Head injury
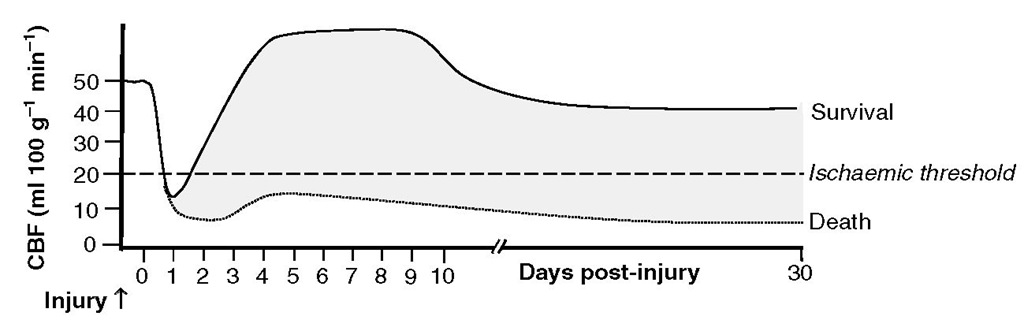
Severe head injury is accompanied by both direct and indirect effects on CBF and metabolism, which show both temporal and spatial variations. Cerebral blood flow may be high, normal or low soon after ictus but is typically reduced. hirty per cent of patients undergoing CBF studies within 6-8 h of a head injury have significant cerebral ischaemia. Global hypoperfusion in these studies was associated with 100% mortality at 48 h, and regional ischaemia with significant deficits. Cerebral blood flow patterns also vary in relation to the time after injury. Initial reductions are replaced, especially in patients who achieve good outcomes, by a period of relative increase in CBF, which towards the end of the first week post-ictus may be replaced (in some patients) by reductions in CBF that are the consequence of vasospasm associated with traumatic subarachnoid haemorrhage (Fig. 2.11). Changes in CBF are non-uniform in the injured brain.Blood flow tends to be reduced in the immediate vicinity of intracranial contusions, and cerebral ischaemia associated with hyperventilation may be extremely regional and not reflected in global monitors of cere-brovascular adequacy.
Fig. 2.11. Spectrum of cerebral blood flow (CBF) patterns following severe head injury. Following an initial period of ischaemia lasting <24 h, CBF begins to rise and may exceed normal values on days 2-4; CBF may fall to subnormal levels at later time points, chiefly due to the presence of vasospasm secondary to traumatic subarachnoid haemorrhage. The CBF levels may never rise in some patients, especially those who have a poor outcome.
Elevations in ICP result in reductions in CPP and cerebral ischaemia, which lead to secondary neuronal injury. here is strong evidence than maintenance of a CPP above 60 mmHg improves outcome in patients with head injury and raised ICP. Traditionally, patients with intracranial hypertension have been nursed head up in an effort to reduce ICP. It is important to realize, however, that such manoeuvres will also reduce the effective MAP at the level of the head and run the risk of reducing CPP. It has been suggested that a 30° head-up elevation may provide the optimal balance by reducing ICP without decreasing CPP.
Hypertensive encephalopathy
Current concepts of the causation of hypertensive encephalopathy are based on the forced vasodilation hypothesis. Severe acute or sustained elevations in MAP overcome autoregulatory vasoconstriction in the resistance vessels and result in forced vasodilation. hese vasodilated vessels, exposed to high intraluminal pressures, leak fluid and protein and result in cerebral oedema, which is multifocal and later diffuse.
Subarachnoid haemorrhage
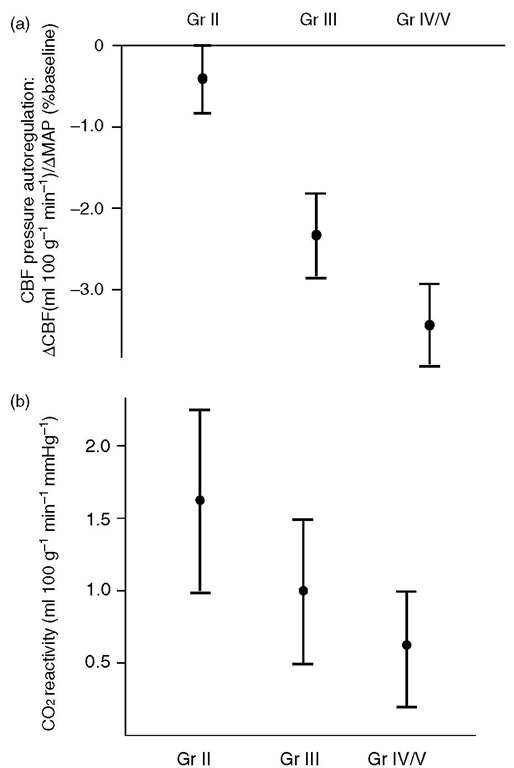
Cerebral autoregulation and carbon dioxide responsiveness are grossly distorted af er subarachnoid haemorrhage (SAH), more so in patients in worse clinical grades (Fig. 2.12). Such patients may be unable to compensate for reductions in MAP produced by anaesthetic agents and develop clinically significant deficits.
Clinically significant vasospasm after SAH occurs in up to 30-40% of patients, typically several days af er the initial bleed, and may be due to one or more of several mechanisms. Nitric oxide may be taken up by haemoglobin in the extravasated blood or be inactivated to peroxynitrite (ONOO) by superoxide radicals (O-) produced during ischaemia and reper-fusion. Alternatively, spasm may be secondary to lipid peroxidation of the vessel wall by various oxidant species including superoxide and peroxynitrite. Other authors have proposed a role for endothelin. Vasospasm tends to be worst in patients with the largest amounts of subarachnoid blood, suggesting that the blood in itself contributes to the phenomenon. Vasospasm is associated with parallel reductions in rCBF and CMRO2 in the regions affected.
Fig. 2.12. Effect of Hunt and Hess grades of aneurysmal subarachnoid haemorrhage on (a) CBF reactivity to changes in PaCO2 and (b) pressure autoregulation.
The clinical impact of late vasospasm has been substantially m odified by the routine use of calcium -channel blockers such as nimodipine, and by the routine use of hypertensive-hypervolaemic-haemodilution (triple H) therapy. Triple H therapy involves the use of colloid administration (with venesection if needed) to increase filling pressures and reduce the haematocrit to 30-35%. If moderate hypertension is not achieved with volume loading, vasopressors and inotropes are used to maintain systolic blood pressures as high as 120-140 mmHg. he hypertensive element of this therapy protects non-autoregulating portions of the cerebral vasculature from hypoperfusion, while the haemodilution improves rheological characteristics of blood and facilitates flow through vessels whose calibre is reduced by spasm. Such interventions have been shown to produce clinically useful improvements in rCBF in regions of ischaemia in the setting of SAH but not in stroke.
Mechanisms of regional cerebral blood flow control
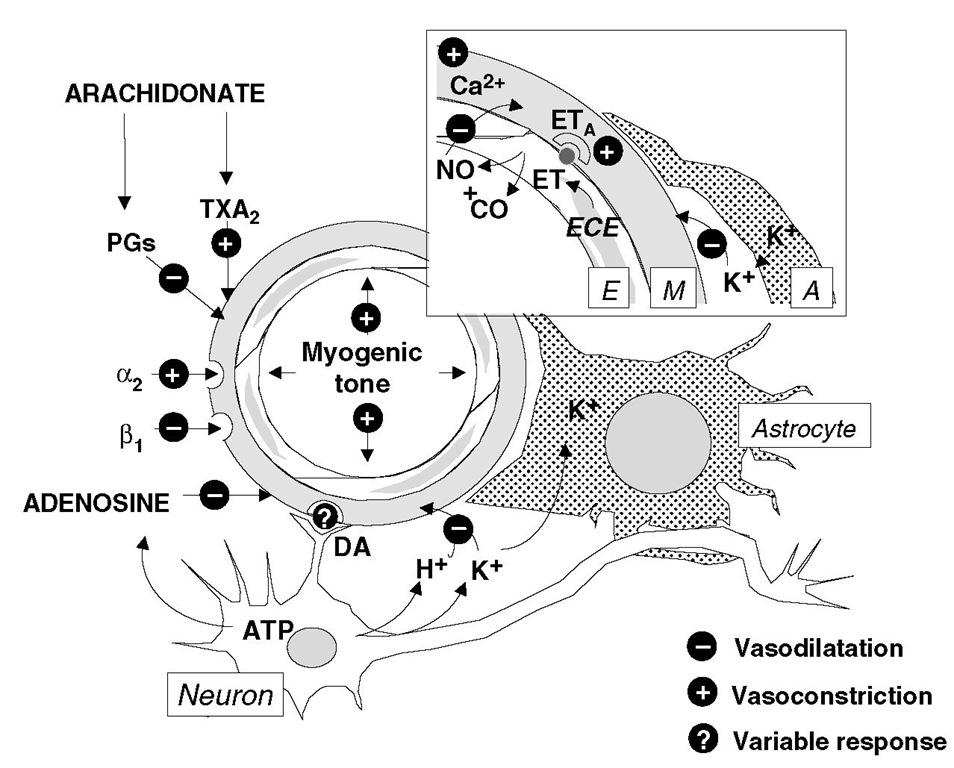
Some of the mechanisms involved in cerebrovascular control are shown in Fig. 2.13 . Several of these have been referred to earlier. In addition, the level of free Ca2+ is important in determining vascular tone, and arachidonate metabolism can produce prostanoids that are either vasodilators (e.g. PGI2) or vasoconstrictive (e.g. TXA– . Endothelin (ET), produced by endothelin-converting enzyme in endothelial cells, balances the vasodilator effects of nitric oxide in a tonic matter by exerting its influences at ETA receptors in the vascular smooth muscle.
Nitric oxide in the regulation of cerebral haemodynamics
Recent interest has focused on the role of NO in the control of cerebral haemodynamics. Nitric oxide is synthesized in the brain from the amino acid l- ar-ginine by the enzyme NO synthase. his form of the enzyme is calmodulin dependent and requires Ca-+ and tetrahydrobiopterin for its activity, and differs from the inducible form of the enzyme that is present in mononuclear blood cells and is activated by cytokines. Under basal conditions, endothelial cells synthesize NO, which diffuses into the muscular layer and, via a cGMP-mediated mechanism, produces relaxation of vessels. here is strong evidence to suggest that NO exerts a tonic dilatory influence on cerebral vessels. It is important to emphasize that data on NO obtained from peripheral vessels cannot always be translated to the cerebral vasculature; for example, some of the endothelium-derived relaxant factor activity in cerebral vessels may be due to compounds other than NO. here is growing evidence that carbon monoxide (CO) produced by haeme oxygenase may be responsible for significant cerebral vasodilation, especially when NO production is reduced. Nitric oxide plays an important role in cerebrovascular responses to functional activation, excitatory amino acids, hypercapnia, ischae-mia and subarachnoid haemorrhage. Furthermore, it may also mediate the vasodilation produced by volatile anaesthetic agents, although other mechanisms, including a direct effect on the vessel wall, cannot be excluded.
Fig. 2.13. Mechanisms involved inthe regulation of rCBF in health and disease. The diagram shows a resistance vessel in the brain in the vicinity of a neuron and an astrocyte (A). E, endothelium; M, muscular layer; PGs, prostaglandins; TXA2, thromboxane A2; ET, endothelin; ECE, endothelin-converting enzyme; ETA, ETa receptor; NO, nitric oxide; CO, carbon monoxide; DA, dopamine. The inset box shows the detail of the vessel wall and adjacent glial cell process. See text for details.
Opioidergic mechanisms
A noxious stimulus (e.g. pain) increases CBF by sympathetic stimulation, which increases MAP and heart rate. However, the increase in CBF is more than that expected by a mere increase in MAP; the proposed mechanisms in animal models are opening of K+ATP channels, the L-arginine-NO pathway and cyclo-oxygenase products or endogenous opiates. Further research is needed to elucidate the exact mechanisms in humans. Endogenous opioid peptides present in cerebral perivascular nerves and in the CSF change in response to stimuli and activate regulatory mechanisms of the cerebral circulation. he ^ and 5 opiate receptors are implicated in the hypoxia- and hypercap-nia-induced cerebral vasodilation.
Neurogenic flow-metabolism coupling
While the last 10 years have focused on flow-metabolism coupling being effected by a diffusible extracellular mediator, there is now accumulating evidence to suggest that dopaminergic neurons may play a major part in such events and, additionally, may control BBB permeability.