Parasympathetic
Hie cerebrovascular parasympathetic innervation is supplied from a variety of sources, which include the sphenopalatine and otic ganglia and small clusters of ganglion cells within the cavernous plexus, Vidian and lingual nerves. Vasoactive intestinal polypeptide (VIP), a potent 28 amino acid polypeptide vasodilator that is not dependent on endothelium-derived relaxant factor, has been localized immunohistochemically within parasympathetic nerve endings, as has nitric oxide synthase, the enzyme that forms nitric oxide from L-arginine.
Although stimulation of parasympathetic nerves does elicit a rise in CBF, there is, like the sympathetic nervous system, little to suggest that cholinergic mechanisms contribute significantly to CBF regulation under physiological conditions; nor are parasym-pathetic nerves involved in the vasodilatory response to hypercapnia. However, chronic parasympathetic denervation increases infarct volume by 37% in rats subjected to permanent MCA occlusion, primarily because of a reduction in CBF under situations when perfusion pressure is reduced. his suggests that para-sympathetic nerves may help to maintain perfusion at times of reduced CBF and may explain in part why patients with autonomic neuropathy, such as diabetes, are at increased risk of stroke.
Sensory nerves and head pain
Hie anatomy of the sensory innervation to the cranium is important for an understanding of the basis of certain types of headache. he only pain-sensitive structures within the cranium are the dura mater, the dural venous sinuses and the larger cerebral arteries (>50 ^m diameter). he structures that lie within the supraten-torial compartment and rostral third of the posterior fossa are innervated predominantly by small myelin-ated and unmyelinated nerve fibres that emanate from the ophthalmic division of the trigeminal nerve (with a small contribution from the maxillary division). he caudal two-thirds of the posterior fossa is innervated by the C1 and C2 dorsal roots. With the exception of midline structures, innervation is strictly unilateral. Although each individual neuron has divergent axon collaterals that innervate both the cerebral vessels and dura mater, the extracranial and intracranial trigemi-nal innervations are separate peripherally. Centrally, however, they synapse onto single interneurons in the trigeminal nucleus caudalis.
Hiis arrangement accounts for the strictly unilateral nature of some types of headache. he pain is poorly localized because of large receptive fields, and is referred to somatic areas.Headache is generally referred to the frontal (ophthalmic) or cervico-occipital (C2) regions and is associated with tenderness in the temporalis and cervical musculature. It is because of this arrangement that tumours in the upper posterior fossa may present with frontal headache and why patients with raised pressure within the posterior fossa and impending herniation of the cerebellar tonsils through the foramen magnum may complain of neck pain and exhibit nuchal rigidity or episthotonic posturing. his arching of the back and extension of the limbs may be mistaken for epilepsy, with potentially serious adverse consequences if diazepam is given due to its risk of causing respiratory depression. Central projections of the trigeminal nerve to the nucleus of the tractus solitarius account for the autonomic responses (sweating, hypertension, tachycardia and vomiting) that may accompany headache.
Sensory nerves form a fine network on the adventitial surface of cerebral arteries. Several neuropeptides, including substance P (SP), neurokinin A (NKA) and calcitonin gene-related peptide (CGRP), are contained within vesicles in the naked nerve endings. All three are vasodilators, while SP and NKA promote plasma protein extravasation and an increase in vascular permeability. Neurotransmitter release can follow both orthodromic stimulation and axon reflex-like mechanisms. Trigeminal perivascular sensory nerve fibres have been found to contribute significantly to the hyperaemic responses that follow reperfusion af er a period of cerebral ischaemia and that accompany acute severe hypertension, seizures and bacterial meningitis. here is now considerable evidence to support the notion that neurogenic inflamm ation in the dura m ater resulting from the release of sensory neuropeptides is the fundamental basis for migraine.
Cerebrospinal fluid pathways
Cerebrospinal physiology and anatomy
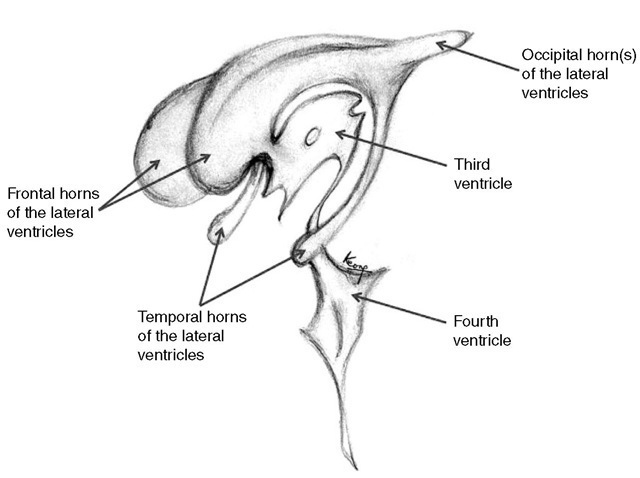
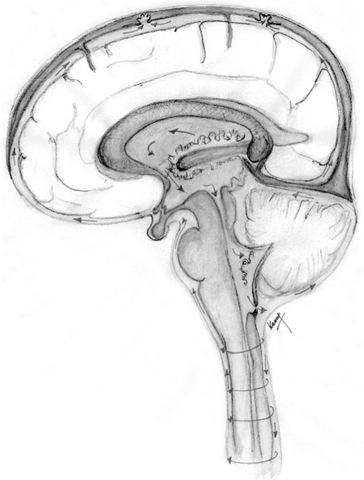
Approximately 80% of the CSF is produced by the chor-oid plexus in the lateral, third and fourth ventricles. Te remainder is formed around the cerebral vessels and from the ependymal lining of the ventricular system. Te rate of CSF production (500-600 ml day-1 in the adult) is independent of intraventricular pressure, until intra-cranial pressure is elevated to the point at which CBF is compromised. Te lateral ventricles are C-shaped cavities within the cerebral hemispheres (Fig. 1.11). Each drains separately into the third ventricle via the foramen of Monro, which is situated just in front of the anterior pole of the thalamus on each side. Te third ventricle is a midline slit, bounded laterally by the thalami and infer-iorly by the hypothalamus. It drains via the narrow aqueduct of Sylvius through the dorsal aspect of the midbrain to open out into the diamond-shaped fourth ventricle. Tis has the cerebellum as its roof and the dorsal aspect of the pons and medulla as its floor. Te fourth ventricle opens into the basal cisterns via a midline foramen of Magendie, which sits posteriorly between the cerebel-lar tonsils and laterally into the cerebellopontine angle via the foraminae of Lushka. Te CSF circulates into the spinal canal and also over the subarachnoid spaces. Figure 1.12 illustrates CSF production and circulation.
Most of the CSF is reabsorbed via the arachnoid villi into the superior sagittal sinus, while some is reabsorbed in the lumbar theca. Te exact mechanism for reabsorption is unknown, although it is thought to be via one-way bulk flow. Reabsorption may be dependent on the pressure differential between the CSF and venous systems, as well as the overlapping arrangement of endothelial cells of the arachnoid villi acting as a valve mechanism. Flow of CSF across the ventricular wall into the brain extracellular space is not an important mechanism under physiological conditions. However, this is seen in acute hydrocephalus as areas of periventricular lucency (PVL), normally at the frontal and occipital horns of the lateral ventricles.
Hydrocephalus
Obstruction to CSF flow results in hydrocephalus. This is divided clinically into communicating and non-communicating types depending on whether or not the ventricular system communicates with the sub-arachnoid space in the basal cisterns. Te distinction between the two is important when considering treatment (see below). Examples of communicating hydrocephalus include subarachnoid haemorrhage (either traumatic or spontaneous, both of which silt up the arachnoid villi), meningitis and sagittal sinus thrombosis. In contrast, aqueduct stenosis, intraventricular haemorrhage and intrinsic tumours are common causes of non-communicating hydrocephalus. Other types of hydrocephalus are recognized such as normal pressure hydrocephalus, arrested hydroceph-alus, long-standing overt ventriculomegaly in adults (LOVA), slit ventricles or unresponsive ventricles and slit ventricle syndrome. Te descriptions, differentiation and investigation of these types are beyond the scope of this topic (but are discussed by Keong et al., 2011).
Fig. 1.11. An illustration of the three-dimensional shape of the ventricular system.
Fig. 1.12. Cerebrospinal fluid production and circulation.
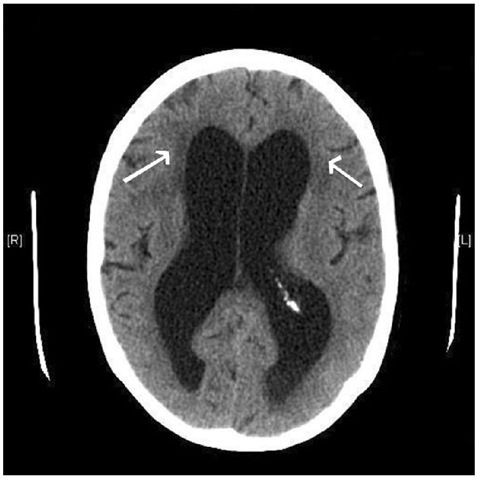
The diagnosis of hydrocephalus is usually made by the appearance on CT or MRI scan (Fig. 1.13). Te features that suggest active hydrocephalus rather than ex vacuo dilation of the ventricular system secondary to brain atrophy are as follows:
• Dilation of the temporal horns of the lateral ventricles (>2 mm width).
• Rounding of the third ventricle or ballooning of the frontal horns of the lateral ventricles.
• Low density surrounding the frontal horns of the ventricles. Tis is caused by transependymal flow of CSF and is known as periventricular lucency. However, in the elderly, this sign may be misleading, as it is also seen after multiple cerebral infarcts.
Fig. 1.13. CThead scan demonstrating dilation of the lateral ventricles and periventricular lucency (arrows).
Management of hydrocephalus
In communicating hydrocephalus, CSF can be drained from either the lateral ventricles or the lumbar theca. Generally, this will involve the insertion of a perm anent indwelling shunt unless the cause of the hydrocephalus is likely to be transient, infection is present or blood within the CSF is likely to block the shunt. Under such circumstances, either an external ventricular or lumbar drain, or serial lumbar punctures may be appropriate. Non-communicating or obstructive hydrocephalus requires CSF drainage from the ventricular system. Lumbar puncture is potentially dangerous because of the risk of coning if a pressure differential is created between the cranial and spinal compartments. A single drainage catheter is adequate if the lateral ventricles communicate with each other (the majority of cases), but bilateral catheters are needed if the blockage lies at the foramen of Monro.
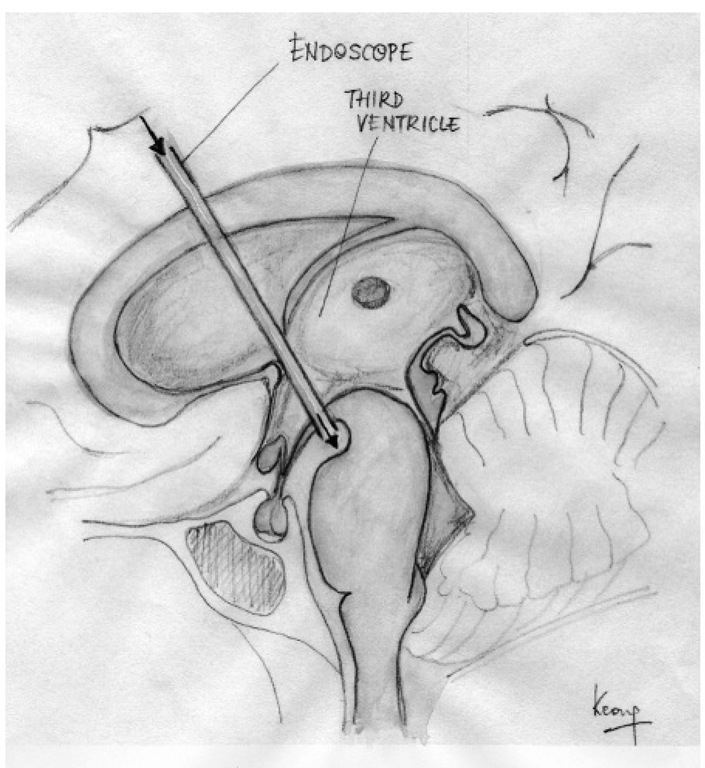
Many forms of non-communicating hydroceph-alus can now be treated by endoscopic third ventricu-lostomy, obviating the need for a prosthetic shunt with its attendant risk of blockage and infection (Fig. 1.14). An artificial outlet for CSF is created in the floor of the third ventricle between the mamillary bodies and the infundibulum via an endoscope introduced through the frontal horn of the lateral ventricle and foramen of Monro. his allows CSF to drain directly from the third ventricle into the basal cisterns, where it emerges between the posterior clinoid processes and basilar artery.
Fig. 1.14. Endoscopic third ventriculostomy.
Spinal anatomy
Assessing stability of the vertebral column
A stable spine is one in which normal movements will not result in displacement of the vertebrae. In an unstable spine, alterations in alignment may occur within movement. Instability can be a result of trauma, infection, tumour, degenerative changes or inflammatory disease. However, the degree of bone destruction or spinal instability does not always correlate with the extent of spinal cord injury.
The anterior column of the spine is formed by the anterior longitudinal ligament, the anterior annulus fibrosus of the intervertebral disc and the anterior part of the vertebral body. he middle column is formed by the posterior longitudinal ligament, the posterior annulus fibrosus of the intervertebral disc and the posterior wall of the vertebral body. he posterior column is formed by the posterior arch and supraspinous and interspinous ligaments, as well as the ligamentum fla-vum. A single column disruption is stable, but a two-or three-column disruption should be managed as a potentially unstable injury until proven otherwise.
Plain radiographs showing normal vertebral alignment in a neutral position do not necessarily indicate stability, and views in flexion and extension may be necessary to assess the degree of ligamentous or bony damage (Fig. 1.15 t . While an unstable spine should generally be maintained in a fixed position, not all movements will necessarily risk compromising neurological function. For example, if a vertebral body has collapsed because of infection or tumour but the posterior elements are preserved, then the spine will be stable in extension, but flexion will increase the deformity and may force diseased tissue or the buckled posterior longitudinal ligament into the spinal canal. he mechanism of the injury has an important bearing on spinal stability after trauma. As a general rule, wedge compression fractures to the spine are stable, but flexion-rotation injuries cause extensive ligamentous damage posteriorly and are therefore unstable.
The spinal cord
Hie adult spinal cord terminates at about the lower border of L1 as the conus medullaris. Below this, the spinal canal contains peripheral nerves known as the cauda equina. Lesions above this level produce upper motor neuron signs and those below it a lower motor neuron pattern. Lesions of the conus itself may produce a mixed picture. A detailed account of the ascending and descending pathways is beyond the scope of this topic but can be found in all standard anatomical texts. However, the following patterns of involvement may be a useful guide.
Extrinsic spinal cord compression
Classically, this produces symmetrical corticospi-nal (‘pyramidal’) involvement, with upper motor neuron weakness below the level of the compression (increased tone, clonus, little or no muscle wasting, no fasciculation, exaggerated tendon reflexes and extensor plantar responses), together with a sensory loss. If, however, the mass is laterally placed, then the pattern may initially be of hemisection of the cord – the Brown-Sequard syndrome. his produces ipsilateral pyramidal weakness, loss of fine touch and impaired proprioception but contralateral impairment of pain and temperature sensation. Examples of intrinsic spinal cord compression include a spinal meningioma or an epidural haematoma.
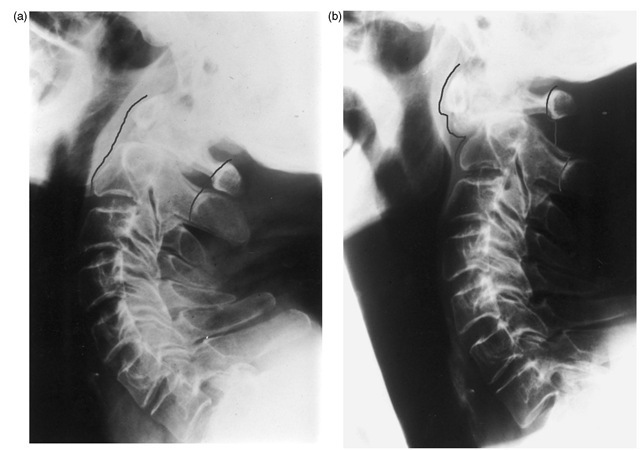
Fig. 1.15. Non-union of a fracture of the dens. (a) Satisfactory vertebral alignment in extension; (b) subluxation of C1 on C2 during neck flexion, resulting in severe spinal canal compromise.
Central cord syndromes
Syringomyelia or intramedullary tumours affecting the cervicothoracic region will first involve the pain and temperature fibres, which decussate near the midline before ascending the lateral spinothalamic tracts. Te result of central cord involvement is therefore a ‘suspended’ sensory loss, with a cape-type distribution of loss of sensitivity to pain in the upper limbs and trunk but with sparing of the lower limbs.
Cauda equina compression
This may result, for example, from a lumbar disc prolapse. Usually, but not invariably, it is accompanied by sciatica, which may be bilateral or unilateral and there may be weakness or sensory loss in a radicular distribution. In addition, there is perineal sensory loss in a saddle distribution, painless retention of urine with dribbling overflow incontinence and loss of anal tone.
Blood supply
The blood supply to the spinal cord is tenuous. Te anterior and posterior spinal arteries form a longitudinal anastomotic channel that is fed by spinal branches of the vertebral, deep cervical, intercos-tals and lumbar arteries. In the neck, there is usually a feeder which comes from the thyrocervical trunk and accompanies either the C3 or C4 root. Te largest radicular artery arises from the lower thoracic or upper lumbar region and supplies the spinal cord below the level of about T4. As in the cervical region, it accompanies one of the nerve roots and is known as the artery of Adamkiewicz. Its position is variable but is generally on the left side (two-thirds of cases) and arises between T10 and T12 in 75% of patients. In 15%, it lies between T5 and T8 and in 10% at L1 or L2.
It has a characteristic hairpin appearance on angiog-raphy because it ascends up the nerve root and then splits into a large caudal and a small cranial branch when it reaches the cord.
Hie artery of Adamkiewicz is vulnerable to damage during operations on the thoracic spine, particularly during excision of neurofibromas or meningiomas, or if an intercostal vessel is divided during excision of a thoracic disc. he artery is also vulnerable to injury during surgery to the descending thoracic aorta, during nephrectomy or even intercostals nerve blocks. Atherosclerotic disease of the radicular artery or prolonged hypotension may induce infarction of the anterior half of the cord up to mid-dorsal level, producing paraplegia, incontinence and spinothalamic sensory loss. However, joint position sense and light touch are preserved. Posterior spinal artery occlusion does not often produce a classic distribution of deficit because the vessel is part of a plexus and the territory of supply is variable. Because the arterial supply to the cord is not readily apparent at operation, surgery to the lower thoracic spine is often preceded by spinal angiography to determine the precise location of the artery of Adamkiewicz. However, this procedure itself carries a very small risk of spinal cord infarction.