Introduction
This topic provides an overview of some of the key neuroanatomical considerations that may impact on neuroanaesthesia and neurointensive care. Te topics and discussions are by no means exhaustive but serve as a platform for further exploration via standard neuroanatomical and neurosurgical texts.
Applied anatomy of the cranium
Anatomical considerations in planning surgical access
There are multiple factors that require consideration when planning an operative approach. All available imaging of the pathology should be reviewed to assess the surgical options. Further imaging, such as angiography or image-guidance sequences, may be appropriate. Where multiple surgical strategies are possible, the decision regarding the operative approach may be influenced by cosmesis, previous surgery and technical preference of the operating surgeon, as well as potential risks. T e most direct route to pathology via the smallest possible exposure may not necessarily produce the best outcome. Other considerations are discussed below.
Pre-operative considerations
The ideal surgical approach to pathology should avoid eloquent areas of the brain in order to minimize the risk of producing further neurological deficit. In cases of extra-axial midline structures, surgical approaches are generally via the non-dominant side. Some areas of the brain, such as the temporal lobe, are more epilepto-genic than others and this also needs to be taken into account. For example, approaching the lateral ventricle via an interhemispheric route through the corpus cal-losum is less likely to induce seizures than an approach via the frontal lobe.
Stereotactic or image-guided methods are useful in planning targets and trajectory, but some will require a form of rigid head fixation. Where pathology is within or adjacent to eloquent brain, pre-operative assessment using functional MRI (fMRI) may be indicated. Awake craniotomy may be the preferred surgical option for such pathology. Te surgical approach will also determine patient positioning. It is important to be aware of particular risk factors of certain positions, for example, air embolism in the sitting position, or venous hypertension if there is excessive rotation of the neck. It is essential that the laterality of the pathology is confirmed before commencing the procedure.
Intraoperative considerations
If stereotactic or image-guidance methods are used, pre-operative planning and patient registration are necessary. Tese methods allow intraoperative navigation to target the lesion and may also assist with identification of resection margins (both soft tissue and bony). However, it must be appreciated that such methods range from among navigation based upon images acquired pre-operatively or intraoperatively to real time images, depending on the technical specification of the system. On-table localization of pathology does offer the option of fashioning a small bone flap directly over the lesion, which may be beneficial for cosmesis. However, a large bone flap is indicated in trauma or in other situations where the brain is swollen or likely to swell post-operatively. Tis provides the opportunity of not replacing the bone flap at the end of the procedure in order to provide a decompression, which reduces intracranial pressure. In this situation, the dura is also left widely opened or only loosely tacked together. A large craniectomy is preferable to a small bony defect because there is less risk that brain herniation through the opening will obstruct the pial vessels at the dural margin and result in ischaemia or infarction of the prolapsing tissue.
In order to access the pathology, brain retraction may be required. A good anaesthetic is fundamental for providing satisfactory operating conditions that minimize the need for retraction. Patient positioning is also crucial to reduce venous pressure (for example, avoiding excessive neck rotation) and, where possible, to take advantage of the effect of gravity. he brain is intolerant of retraction, particularly if it is prolonged or over a narrow area. In addition to the risk of brain injury, inappropriate retraction may produce brain swelling or intraparenchymal haemorrhage. Early cerebrospinal fluid (CSF) drainage is another manoeuvre that may assist surgical exposure. his may be achieved by microsurgical dissection into various CSF cisterns at the operative site, access to lateral ventricles by means of a direct ventricular tap or via lumbar CSF drainage. Where appropriate, cortical incisions are made through the sulci rather than the gyri. Preservation of the draining veins is another factor that should be considered in order to minimize post-operative swelling and reduce the risk of venous infarction.
An appropriate size of craniotomy is fundamental in order to achieve good visualization of pathology while minimizing the need for retraction. In addition to this, various extended cranio-facial and skull base exposures have been developed to improve access to specific areas. Examples include the translabyrinthine approach to a large acoustic neuroma to minimize displacement of the cerebellum, or osteotomy of the zygomatic arch in the subtemporal approach to achieve good visualization of a basilar apex aneurysm.
Key aspects of functional neuroanatomy
Surface markings of the brain
The precise position of intracranial structures varies, but a rough guide to major landmarks is as follows. Draw an imaginary line across the top of the calvaria in the midline between the nasion and inion (external occipital protuberance). he Sylvian fissure runs in a line from the lateral canthus to three-quarters of the way from nasion to inion. he central sulcus (separating the motor from the sensory cortex) lies 2 cm behind the midpoint from nasion to inion and joins the Sylvian fissure at a point vertically above the condyle of the mandible.
Brain structure and function
The functional relevance of various cortical areas in the brain, such as language, has been well described, but it is important to note that these areas can vary considerably. However, disorders of different lobes of the brain generally produce characteristic clinical syndromes, dependent not only on site but also side. In terms of laterality, 93-99% of all right-handed patients are left -hemisphere dominant, as are the majority of left-handers and those who are ambidextrous (ranging from 50 to 92% in various studies). Large intracranial mass lesions may present with symptoms or signs of raised intracranial pressure. However, small mass lesions in anatomically eloquent areas may present early with specific focal deficits, particularly in cases of haemorrhage. Epilepsy may also occur as the presenting symptom.
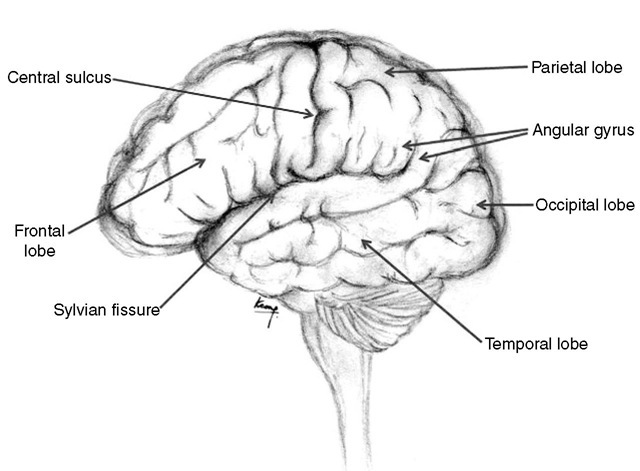
Surgical resections may be undertaken in eloquent parts of the brain either by remaining within the confines of the disease process (intracapsular resection) or by employing some form of cortical mapping. his involves either cortical stimulation during awake cra-niotomy or pre-operative fMRI, which is then linked to an intraoperative image-guidance system. However, a good grasp of neuroanatomy is essential both in the operating room as well as the pre-operative stage in terms of assessing the relative likelihood of pathology causing the clinical symptoms and signs. A general discussion of the functional significance of the cerebral and cerebellar hemispheres is set out below. Figure 1.1 illustrates the lobes of the brain.
Frontal lobes
The frontal lobes are the cerebral hemispheres anterior to the Rolandic fissure (central sulcus; Fig. 1.11 . Important areas within the frontal lobes are the motor strip, Broca’s speech area (in the dominant hemisphere) and the frontal eye fields. Patients with bilateral frontal lobe dysfunction present typically with personality disorders, dementia, apathy and disinhibition. he anterior 7 cm of one frontal lobe can be resected without significant neurological sequelae, providing the contralateral hemisphere is normal. his may account for the relative late presentation and large size of some frontal lesions. Resections more posterior than this in the dominant hemisphere are likely to damage the anterior speech area.
Fig. 1.1. Lobes of the brain.
Temporal lobe
The temporal lobe lies anteriorly below the Sylvian fissure and becomes the parietal lobe posteriorly at the angular gyrus (Fig. 1.1). Its medial border is the uncus and is of particular clinical importance because it overhangs the tentorial hiatus adjacent to the midbrain. When intracranial pressure rises in the supratentorial compartment, it is the uncus of the temporal lobe that transgresses the tentorial hiatus, compressing the third nerve, midbrain and posterior cerebral artery. Tis is described as ‘uncal herniation’ to distinguish it from herniation of the tonsils through the foramen magnum (coning). In around 90% of cases, uncal herniation will produce dilation of the pupil on the same side as the pathology. In the remainder, it is a false localizing sign, where shift of the midbrain compresses the contralateral third nerve against the tentorial hiatus. It is also important to note another herniation syndrome, Kernohan’s notch, where a space-occupying lesion produces midline shift of the midbrain and compresses the contralateral cerebral peduncle against the tentorium. Tis compression causes an ischaemic infarct in the corticospinal tract, resulting in a motor deficit ipsilat-eral to the pathology.
The temporal lobe has many roles including memory, the cortical representation of olfactory, auditory and vestibular information, some aspects of emotion and behaviour, Wernicke’s speech area (in the dominant hemisphere) and parts of the visual field pathway. Like the frontal lobe, lesions in the temporal lobe may present with memory impairment or personality change. Seizures are common because structures in this lobe are particularly epileptogenic. Amygdalohippocampectomy with or without temporal lobectomy may be required for intractable forms of epilepsy with proven mesial temporal sclerosis on imaging. Temporal lobe seizures may be associated with vivid aura phenomena linked to the function of the temporal lobe (e.g. olfactory, auditory or visual hallucinations, unpleasant visceral sensations, bizarre behaviour or deja vu).
The anterior portion of one temporal lobe (approximately at the junction of the Rolandic and Sylvian fissures) may be resected with low risk of neurodisability. Generally, this amounts to 4 cm of the dominant lobe or 6 cm of the non-dominant lobe. Te upper part of the superior temporal gyrus is generally preserved to protect the branches of the middle cerebral artery (MCA) lying in the Sylvian fissure. More posterior resection may also damage the speech area in the dominant hemisphere. Care is needed if resecting the medial aspect of the uncus because of its proximity to the optic tract. In some patients undergoing temporal lobectomy, it may be appropriate to perform an fMRI investigation to confirm laterality of language and to establish whether the patient is likely to suffer significant memory impairment as a result of the procedure. Previously, patients would have undergone the Wada test prior to surgery. his investigation involves selective catheterization of each internal carotid artery in turn. While the hemisphere in question is anaesthetized with sodium amytal (effectiveness is confirmed by the onset of contralateral hemiplegia), the patient’s ability to speak is evaluated. hey are then presented with a series of words and images that they are asked to recall once the hemiparesis has recovered, thereby assessing the strength of verbal and non-verbal memory in the contralateral hemisphere.
Parietal lobes
These extend from the Rolandic fissure to the parieto-occipital sulcus posteriorly and to the temporal lobe inferiorly. he dominant hemisphere shares speech function with the adjacent temporal lobe, while both sides contain the sensory cortex and visual association areas. Parietal lobe dysfunction may produce cortical sensory loss or sensory inattention. In the dominant hemisphere, the result is dysphasia. Dysfunction in the non-dominant hemisphere produces dyspraxia (e.g. difficulty dressing, using a knife and fork) or difficulty with spatial orientation. Impairment of the visual association areas may give rise to visual agnosia (inability to recognize objects) or to alexia (inability to read).
Occipital lobes
Lesions within the occipital lobe typically present with a homonymous field defect without macular sparing. Visual hallucinations (flashes of light, rather than the formed images that are typical of temporal lobe epilepsy) may also be a feature. Resection of the occipital lobe will result in a contralateral homonymous hemian-opia. he extent of resection is restricted to 3.5 cm from the occipital pole in the dominant hemisphere because of the angular gyrus, where lesions can produce dyslexia, dysgraphia and acalculia. In the non-dominant hemisphere, up to 7 cm may be resected.
Cerebellum
Hie cerebellum consists of a group of midline structures, the lingula, vermis and flocculonodular lobe, and two laterally placed hemispheres. Lesions affecting midline structures typically produce truncal ataxia, which may make it difficult for the patient to stand or even to sit. Obstructive hydrocephalus is common. Invasion of the floor of the fourth ventricle by tumour may give rise to vomiting or cranial nerve dysfunction. Lesions within the hemispheres usually cause ipsilateral limb ataxia. Vertigo may result from damage to the vestibular reflex pathways. Nystagmus is typically the result of tnvolvement of the flocculo-nodular lobe. Other features associated with disorders of the cerebellum include hypotonia, dysarthria and pendular reflexes.
Surgical anatomy of the cerebral circulation
Arterial
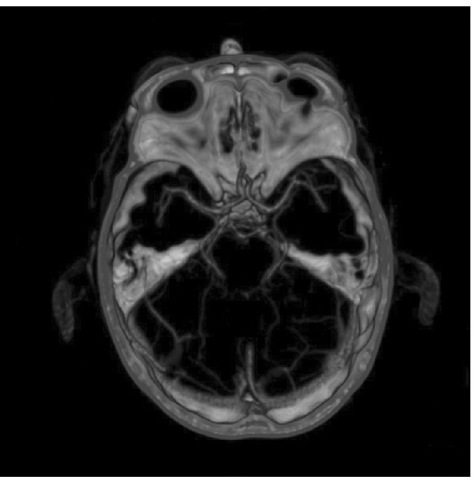
Hie cerebral circulation is made up of two components. he anterior circulation is fed by the internal carotid arteries, while the posterior circulation derives from the vertebral arteries (the vertebrobasilar circulation). he arterial anastomosis in the suprasellar cistern is named the ‘circle of Willis’ after homas Willis (Fig. 1.2), who published his dissections in 1664, with illustrations by the architect Sir Christopher Wren. his section is based on detailed accounts of the normal and abnormal anatomy of the cerebral vasculature, as described by Yasargil (1984) and Rhoton (2003).
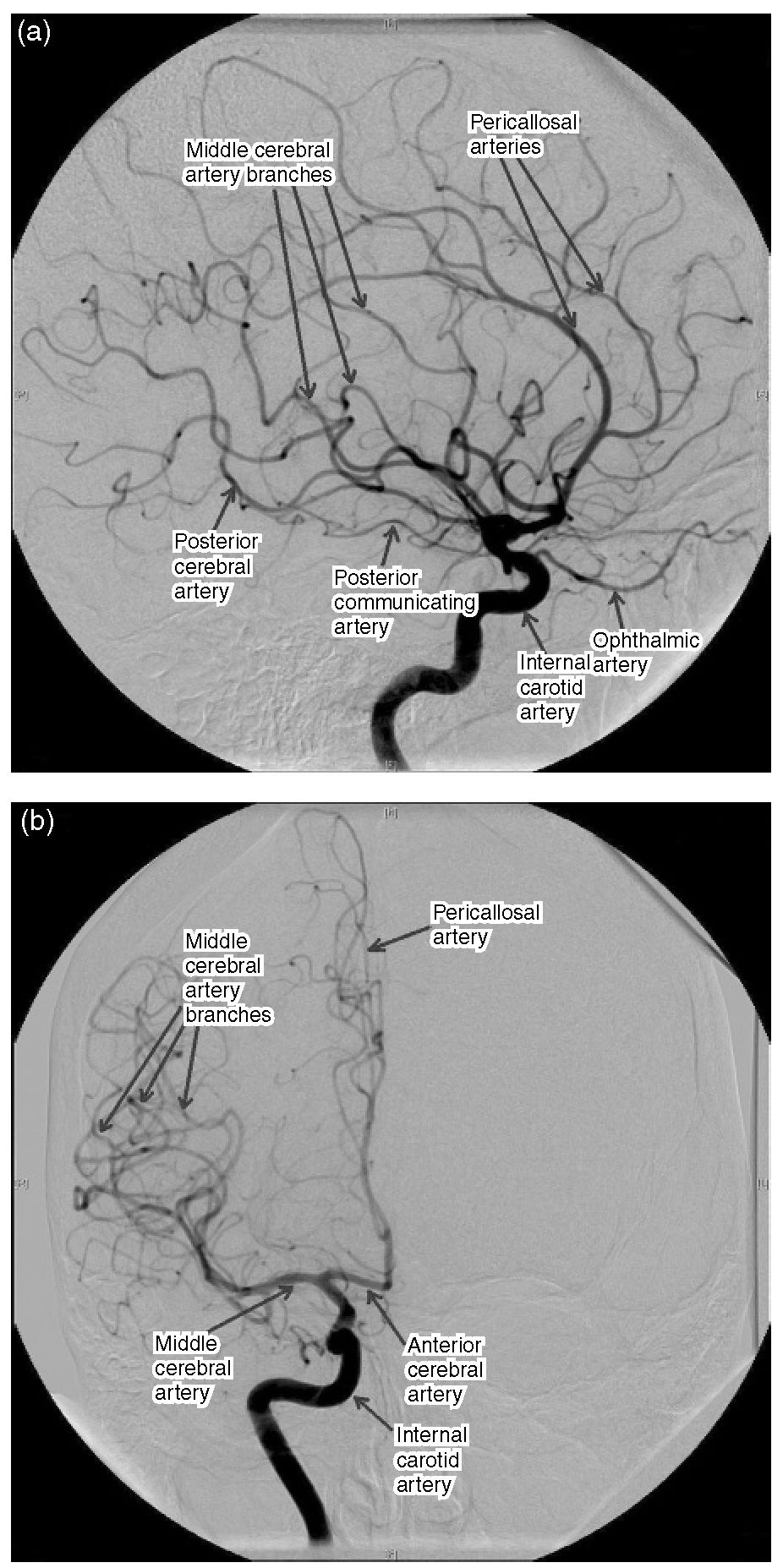
Hie internal carotid artery (ICA) has no branches in the neck but gives off two or three small vessels within the cavernous sinus before entering the cranium just medial to the anterior clinoid process. It gives off the ophthalmic and posterior communicating arteries (PComA) before reaching its terminal bifurcation, where it divides to become the anterior and middle cerebral arteries (Fig. 1.3). Te anterior choroidal artery, the blood supply to the internal capsule, generally arises from the ICA nearer the origin of the PComA than the carotid bifurcation. It m ay arise as two separate arteries or as a single artery that divides into two trunks. Te anterior cerebral artery (ACA) passes over the optic nerve and is connected with the vessel of the opposite side in the interhemispheric fissure by the anterior communicating artery (AComA). Te segment of the anterior cerebral artery proximal to the AComA is known as the A1 segment.
Fig. 1.2. CT angiogram oft he circle of Willis.
Fig. 1.3. Subtraction angiogram ofthe internal carotid circulation. (a) Lateral projection; (b) anteroposterior projection.
Te distal ACA has four segments named according to their location in relation to the corpus callosum, the A2 (infracallosal), A3 (pre-callosal), A4 (supracallosal) and A5 (post-callosal) segments. Te term pericallosal artery refers to the portion of the ACA beyond the A1 and therefore includes all the segments beyond that. In addition, the A2 also branches into the callosal marginal artery, which is variable in its presence and origin. Te ACA supplies the orbital surface of the frontal lobe and the medial surface of the frontal lobe and the medial surface of the hemisphere above the corpus cal-losum back to the parieto-occipital sulcus. It extends onto the lateral surface of the hemisphere superiorly, where it meets the territory supplied by the MCA. Te motor and sensory cortex to the lower limb are within the territory of supply of the ACA.
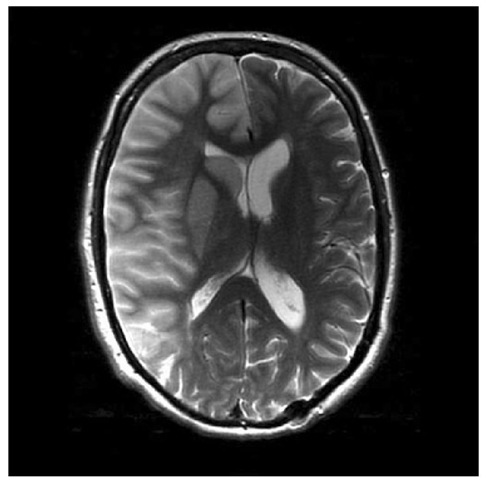
Te MCA is the larger of the two terminal branches of the ICA. Te MCA is divided into four segments, the M1 (sphenoidal), M2 (insular), M3 (opercular) and M4 (cortical) segments. Te M1 segment begins at the origin of the MCA and passes laterally behind the sphenoid ridge and turns 90° at the genu. Te M2 segment begins at the genu and gives off fronto-temporal branches before reaching the insula. Te M3 segment begins at the insula and ends at the surface of the Sylvian fissure, giving off further branches whilst following a tortuous course in the process. Te M4 segment refers to the branches to the lateral cerebral convexity. Te MCA is responsible for the blood supply to most of the lateral aspect of the hemisphere, with the exception of the superior frontal (supplied by the ACA) as well as the inferior temporal gyrus and the occipital cortex (supplied by the posterior cerebral artery, PCA) (Fig. 1.4). Within its territory of supply are the internal capsule, speech and auditory areas, and the motor and sensory areas for the opposite side, with the exception of the lower limbs, which are supplied by the ACA.
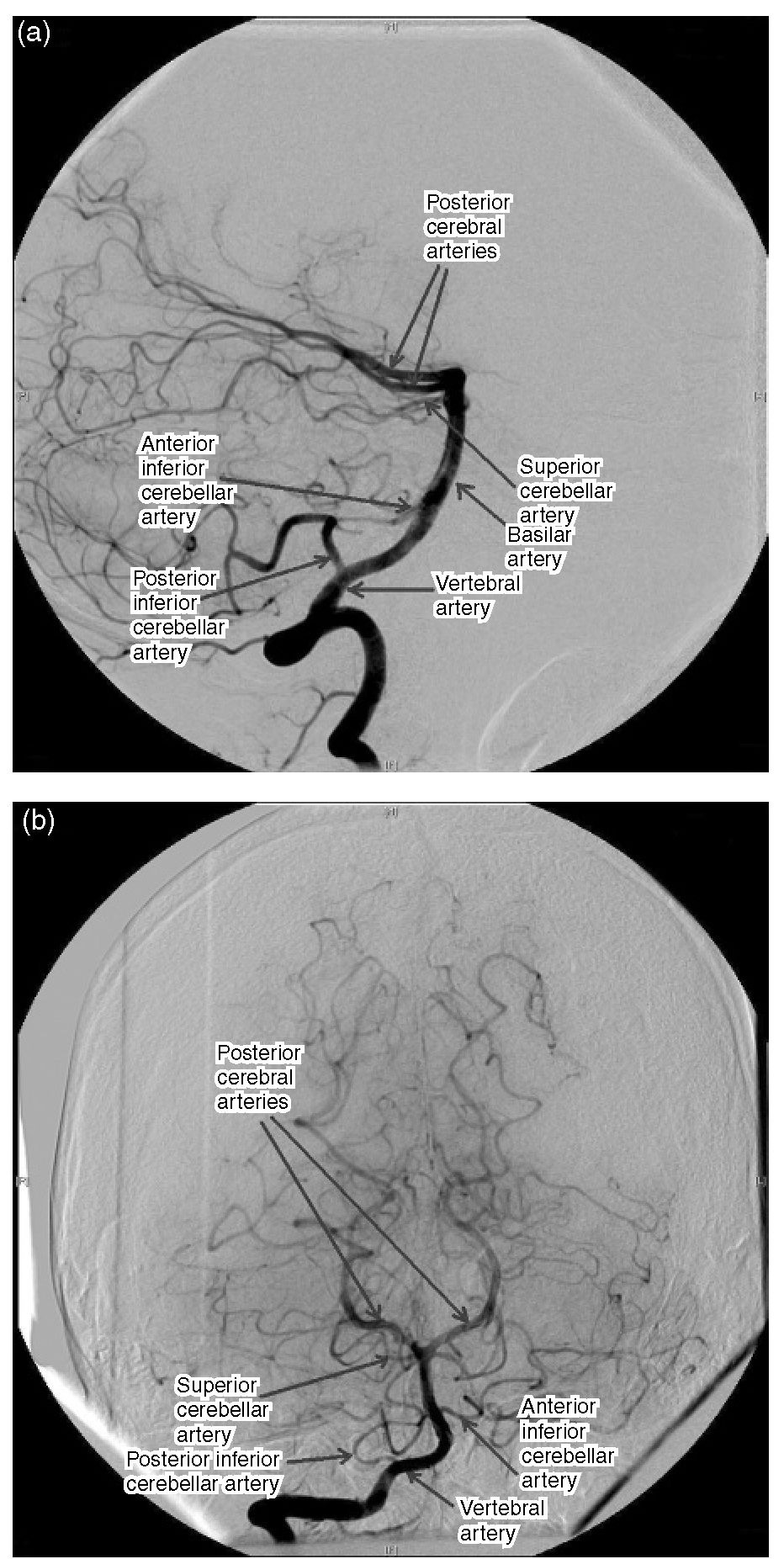
Te PComA arises from the posteromedial boundary of the ICA midway between the origin of the ophthalmic artery and the terminal bifurcation. Te PComA and the proximal PCA form the posterior part of the circle of Willis. Embryologically, the PComA becomes the PCA, but, in the adult, the PCA is a branch of the basilar system. However, the PComA may remain the major origin of the PCA and this is termed a ‘fetal’ PComA. Te posterior circulation comprises the vertebral arteries, which join at the clivus to form the basilar artery. his gives off multiple branches to the brainstem and cerebellum before the bifurcation of the basilar artery near the level of the posterior clinoids to become the PCAs. he PComA and PCA join at the lateral margin of the interpeduncular cistern, thus completing the circle of Willis and connecting the anterior and posterior circulations (Fig. 1.5).
Fig. 1.4. Right middle cerebral artery (MCA) territory pathology. MRI brain scan demonstrating oedema following an infarct in the MCA territory.
Hie PCA is divided into four segments, P1 (pre-communicating), P2 (post-communicating), P3 (quad-rigeminal) and P4 (cortical). he PCA gives off three kinds of branches: (i) central perforating branches to the diencephalon and midbrain; (ii) ventricular branches to the choroid plexus and walls of the lateral and third ventricles and adjacent structures; and (iii) cerebral branches to the cerebral cortex and sple-nium of the corpus callosum. he P1 segment extends from the basilar bifurcation to the junction with the PComA. he P2 lies in the crural and ambient cisterns and then terminates lateral to the posterior edge of the midbrain. he P2 is divided into anterior (P2A) and posterior (P2P) parts. he artery may be occluded as it crosses the tentorial hiatus when intracranial pressure is high (Fig. 1.6). he P3 segment courses from the lateral edge of the midbrain and ambient cistern to the lateral part of the quadrigeminal cistern and ends at the calcarine sulcus. he P4 segment begins at the calcarine sulcus and continues as branches to the cortical surface. Its territory of supply is the inferior and inferolateral surface of the temporal lobe and the inferior and most of the lateral surface of the occipital lobe. he contralateral visual field lies entirely within its territory.
Fig. 1.5. Subtraction angiogram oft he vertebral circulation. (a) Lateral projection; (b) anteroposterior projection.