Arterial anomalies
In post-mortem series, a fully developed arterial circle of Willis exists in about 96% of cadavers, although the communicating arteries will be small in some.
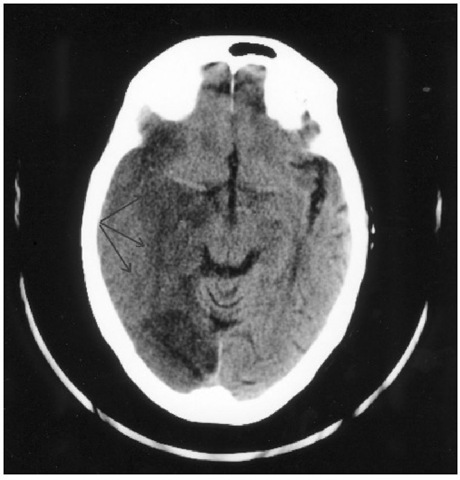
Fig. 1.6. CT head scan showing extensive infarction (lowdensity) in the territory of the posterior cerebral artery (arrows). This was the result of compression of the vessel at the tentorial hiatus due to uncal herniation.
Because haemodynamic anomalies are associated with an increased risk of berry aneurysm formation, an incomplete circle of Willis is likely to be more common in neurosurgical patients than in the general population.
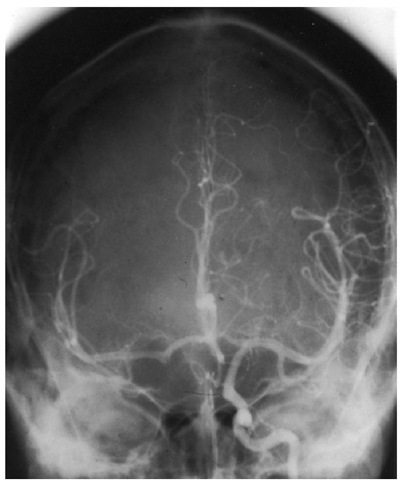
Hypoplasia or absence of one or more of the communicating arteries can be particularly important at times when one of the major feeding arteries is temporarily occluded. his is an important consideration for neurovascular procedures such as during carotid end-arterectomy or when gaining proximal control of a ruptured intracranial aneurysm. Under such conditions, the circle of Willis cannot be relied upon to maintain adequate perfusion to parts of the ipsilateral or contra-lateral hemisphere. his situation will be compounded by atherosclerotic narrowing of the vessels or by systemic hypotension. he areas particularly vulnerable to ischaemia are the watersheds between vascular territories. Some estimate of flow across the ACom A can be obtained angiographically by the cross-compression test. During contrast injection, the contralateral carotid is compressed in the neck, thereby reducing distal per-fusion and encouraging flow of contrast from the ipsi-lateral side (Fig. 1.7). Transcranial Doppler provides a more quantitative assessment. Trial balloon occlusion in the conscious patient may be indicated for further evaluation of the presence of cross-flow and tolerance of permanent occlusion.
Fig. 1.7. The cross-compression test. Contrast has been injected into the left internal carotid artery while the right is occluded by external compression in the neck. This examination demonstrates good cross-filling of the distal vessels on the right from the left. The AComA and A1 segments are patent. However, this test alone is not a reliable way of determining that neurodisability will not ensue if the contralateral internal carotid artery is permanently occluded.
Hie A1 segments frequently vary in size (in 60-80% of patients). In approximately 5% of the population, one A1 segment will be severely hypoplastic or aplastic. he AComA is very variable in nature, having developed embryologically from a vascular network. It exists as a single channel in 75% of subjects but may be duplicated or occasionally absent (2%). he PComA is <1 mm in diameter in approximately 20% of patients. In almost 25% of people, the PComA is larger than the P1 segment and the PCAs are therefore supplied primarily (or entirely) by the internal carotid rather than the vertebral arteries. Because the posterior cerebral artery derives embryologically from the internal carotid artery, this anatomical variant is known as a persistent fetal-type posterior circulation (as described above).
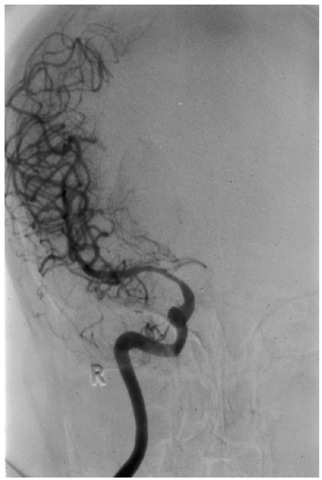
If both the AComA and PComA are hypoplastic, then the middle cerebral territory is supplied only by the ipsilateral internal carotid artery (the so-called ‘isolated MCA’; Fig. 1.8). Such a patient will be very vulnerable to ischaemia if the internal carotid is temporarily occluded during surgery. Should it be necessary to occlude the internal carotid artery permanently, for example in a patient with an intracavernous aneurysm, some form of bypass graft will be required. Usually this is between the superficial temporal artery and a branch of the MCA (an extracranial-intracranial artery (EC-IC) bypass).
Fig. 1.8. Vasospasm in the A1 segment due to an AComA aneurysm. Only the right anteroposterior (MCA) territory fills following right internal carotid artery (ICA) angiography. In this instance, the circle of Willis would be unable to maintain right MCA blood flow if perfusion were to be reduced in the ipsilateral ICA. R, right side.
The small perforating vessels that arise from the circle of Willis to enter the base of the brain are known as the central rami. Tose from the anterior and middle cerebral arteries supply the lentiform and caudate nuclei and internal capsule, while those from the communicating arteries and posterior cerebrals supply the thalamus, hypothalamus and mesencephalon. Damage to any of these small perforators at surgery may result in significant neurological deficit.
Microscopic anatomy
Cerebral vessels are different from their systemic muscular counterparts in that they possess only a rudimentary tunica adventitia. Tis is particularly relevant to subarachnoid haemorrhage. Whereas a clot surrounding a systemic artery will not result in the development of delayed vasospasm, it is likely that the lack of an adventitia allows blood breakdown products access to smooth muscle of the tunica media of the cerebral vessels, thereby giving rise to late constriction.
A second microscopic difference from systemic vessels is that the tunica media of both large and small cerebral arteries has its muscle fibres orientated cir-cumferentially. Tis results in a point of potential weakness at the apex of vessel branches and may lead to aneurysm formation. Approximately 85% of berry aneurysms develop in the anterior circulation.
Venous
Cephalic venous drainage also differs from many other vascular beds in that it does not follow the arterial pattern. Tere are superficial and deep venous systems that, like the internal jugular veins, are valveless (Fig. 1.9 ). Tis is the basis for nursing patients with raised intracranial pressure such as traumatic brain injury and subarachnoid haemorrhage at a slight head elevation (30°)i Anastomotic venous channels allow communication between intracranial and extracranial tissues via diploic veins in the skull. Tese may allow infection from the face or paranasal air sinuses to spread to the cranium, resulting in subdural empyema, cerebral abscess or a spreading cortical venous or sinus thrombosis.
The general pattern for venous drainage of the hemispheres is into the nearest venous sinus. Te superior sagittal sinus occupies the convex margin of the falx and is triangular in cross-section. Because of its semi-rigid walls, the sinus does not collapse when venous pressure is low, resulting in a high risk of air embolism during surgery if the sinus is opened with the head elevated. Venous lakes are occasionally present within the diploe of the skull adjacent to the sinus, and can result in excessive bleeding or air embolus when a craniotomy flap is being turned.
The lateral margin of the superior sagittal sinus contains arachnoid villi responsible for the reabsorption of CSF into the venous circulation. It begins at the floor of the anterior cranial fossa at the crista galli and extends back in the midline, increasing progressively in size, until it reaches the level of the internal occipital protuberance. Here it turns to one side, usually the right, as the transverse sinus. he straight sinus turns to form the opposite transverse sinus at this point. An anastomosis of variable size connects the two and is known as the confluence of the sinuses or torcular Herophili.
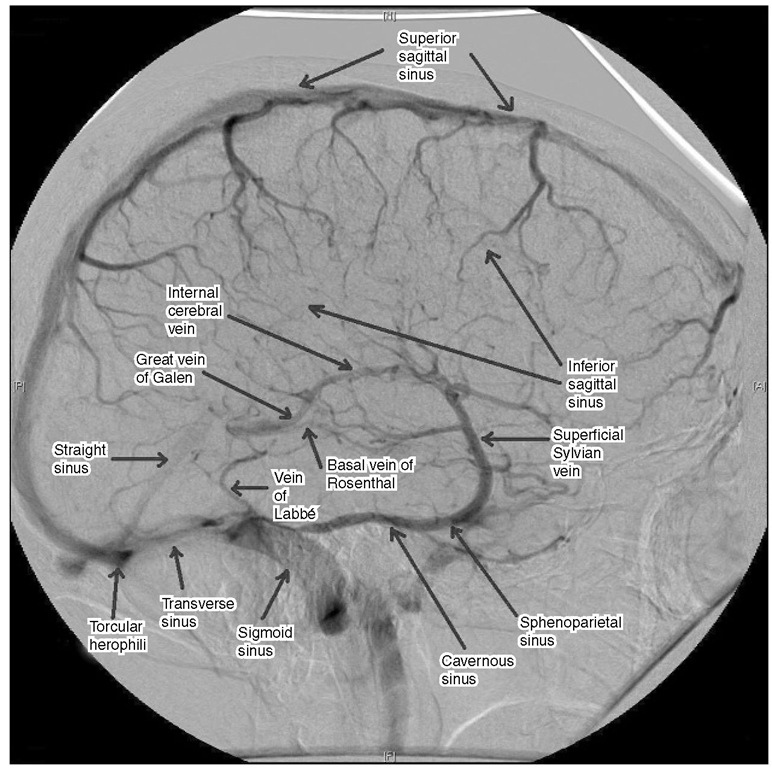
Fig. 1.9. The major superficial and deep venous drainage of the brain on venous phase angiography.
Hie basal ganglia and adjacent structures drain via the internal cerebral veins, which lie in the roof of the third ventricle, and the basal veins, which pass around the cerebral peduncles. he internal cerebral and basal veins join to form the great cerebral vein of Galen beneath the splenium of the corpus callosum. his short vein joins the inferior sagittal sinus (which runs in the free edge of the falx) to form the straight sinus.
Hie superior cerebral veins (usually 8-12 in number) lie beneath the arachnoid on the surface of the cerebral cortex and drain the superior and medial surface of the hemisphere into the superior sagittal sinus. To do this, they must bridge the subdural space (hence the alternative name of ‘bridging veins’). If the hemisphere is atrophic and therefore relatively mobile within the cranium, these veins are likely to be torn by even minor head injury, giving rise to chronic subdural haematoma. A large acute subdural haematoma may also displace the hemisphere sufficiently to avulse the bridging veins, provoking brisk venous bleeding from multiple points in the sinus when the clot is evacuated. Tearing of bridging veins may also occur during or early after neurosurgical procedures in which there has been excessive shrinkage of the brain or loss of CSF. his phenomenon is thought to account for some cases in which post-operative haemorrhage develops in regions remote from the operative site.
Although venous anastomoses exist on the lateral surface of the hemisphere, largely between the superior anastomotic vein (draining upwards in the central sulcus to the superior sagittal sinus – the vein of Trolard), the Sylvian vein (draining downwards in the Sylvian fissure to the sphenoparietal sinus) and the angular or inferior anastomotic vein (draining via the vein of Labbe into the transverse sinus), sudden occlusion of large veins or a patent venous sinus may result in brain swelling or even venous infarction. As a general rule, the anterior one-third of the superior sagittal sinus may be ligated, but only one bridging vein should be divided distal to this if complications are to be avoided. If the sinus has been occluded gradually, for example by a parasagittal meningioma, then there is time for venous collaterals to develop. However, it then becomes all the more important that these anas-tomotic veins are not divided during removal of the tumour. Venous-phase angiography is particularly useful in planning the operative approach to tumours adjacent to the major venous sinuses or to the vein of Galen and thereby determining whether the sinus is completely occluded and can be resected en bloc with the tumour or whether the sinus is patent and requires reconstruction.
Innervation of the cerebral vasculature and neurogenic influences of cerebral blood flow
Sympathetic
The superior cervical ganglion largely supplies sympathetic innervation to the cerebral vasculature. In addition to the catecholamines, sympathetic nerve terminals contain another potent vasoconstrictor, neuropeptide Y. Tis 36 amino acid neuropeptide is found in abundance in both the central and peripheral nervous systems. Only minor (5-10%) reductions in cerebral blood flow (CBF) accompany electrical stimulation of sympathetic nerves, far less than that seen in other vascular beds. Although feline pial arterioles vasoconstrict in response to topical norepinephrine and the response is blocked by the a-blocker phenoxy-benzamine, application of the latter alone at the same concentration has no effect on vessel calibre. Tis and other observations from denervation studies indicate that the sympathetic nervous system does not exert a significant tonic influence on cerebral vessels under physiological conditions. Te sympathetic innervation also does not contribute to CBF regulation under conditions of hypotension or hypoxia.
However, Harper and colleagues (1972) observed that sympathetic stimulation does produce a profound fall in CBF if cerebral vessels have been dilated by hyper-capnia. From this study came the ‘dual-control’ hypothesis, which proposed that the cerebral circulation comprises two resistances in series. Extraparenchymal vessels are thought to be regulated largely by the autonomic nervous system, while intraparenchymal vessels are responsible for the main resistance under physiological conditions and are governed primarily by intrinsic metabolic and myogenic factors.
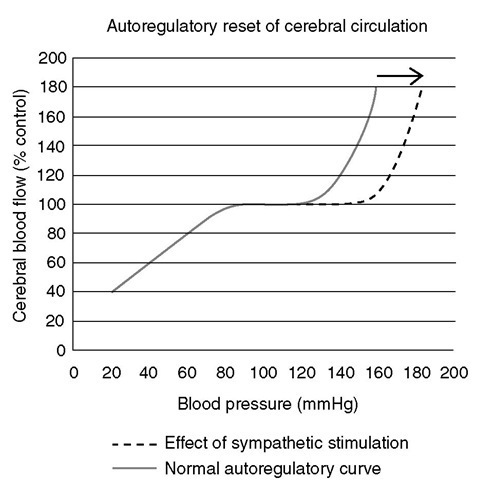
Sympathetic innervation has been shown to exert a significant influence on cerebral blood volume and protect the brain from the effects of acute severe hypertension. When blood pressure rises above the limits of autoregulation, activation of the sympathetic nervous system moderates the anticipated rise in CBF and reduces the plasma protein extravasation that follows breakdown of the blood-brain barrier. Te autoregu-latory curve is ‘reset’ such that both the upper and lower limits are raised. Tis is an important physiological mechanism by which the cerebral vasculature is protected from injury during surges in arterial blood pressure (Fig. 1.10). While cerebral vessels escape from the vasoconstrictor response to sympathetic stimulation under conditions of normotension, this does not occur during acute hypertension. It also follows from this that CBF is better preserved by drug-induced than haemorrhagic hypotension for the same perfusion pressure, because circulating catecholamine levels are high in the case of the latter.
Fig. 1.10. Autoregu latory reset of the cerebral circu lation. The normal curve illustrates the maintenance of cerebral blood flow as arterial blood pressure changes. Stimulation of the sympathetic nervous system results in a shift of the curve to the right, thereby protecting the cerebral vasculature from injury due to surges in blood pressure.
Sympathetic nerves are also thought to exert trophic influences on the vessels that they innervate. Sympathetectomy reduces the hypertrophy of the arterial wall that develops in response to chronic hypertension. Denervation has been shown to increase the susceptibility of stroke-prone spontaneously hypertensive rates to bleed into the cerebral hemisphere, which had been sympathectomized.