Introduction
Ischaemic and traumatic brain injuries are among the most common and important causes of disability and death worldwide. he end point of all cerebral injuries, such as stroke, global cerebral ischaemia during cardiac arrest, cardiac, vascular or brain surgery or head trauma, is the inadequate supply of the brain with oxygen and/or glucose, which triggers a characteristic pathophysiological cascade leading to neuronal death. Multiple neuroprotective strategies have been developed blocking one or more steps along this cascade. This topic provides an overview of the mechanisms leading to neuronal cell death and the most important neuroprotective strategies.
Mechanisms of neuronal injury
Aetiology of neuronal injury
Cerebral ischaemia and/or hypoxia may occur as a consequence of shock, respiratory failure, vascular stenosis or occlusion, vasospasm, neurotrauma or cardiac arrest. hese ischaemic/hypoxic insults evoke a cascade of pathophysiological processes that will result in neuronal damage, because the function and integrity of the brain depends on a constant supply of oxygen and glucose.
Primary and secondary lesions
Neurological outcome after cerebral injury is determined by two factors, the primary and the secondary injury. Primary injury is caused directly by the impact of energy or the initial cerebral hypoxic/hypogly-caemic event (core of infarction), leading to immediate cell loss. he incidence and dimension of primary damage will be minimized only by preventative measures (e.g. treatment of hypertension, diabetes mellitus or hyperlipidaemia and the use of helmet or air bags). Secondary brain damage represents the consecutive pathological processes initiated at the moment of injury with delayed clinical presentation. Within the first days after injury, these pathophysiological processes lead to an expansion of the primary lesion into challenged but still viable tissue. he area at risk of secondary brain damage is called the ‘penumbra’ and can account for up to 50% of the volume that later progresses to damage. he penumbra is a region of hypoperfused tissue surrounding the core, where the blood flow ranges between the thresholds of cell viability and functional activity. Metabolic derangement and neurochemical events within the penumbra lead to cell death unless reperfusion restores the metabolism/flow mismatch in the threatened area. he factors contributing to secondary brain damage in the penumbra represent the targets of potential neuroprotective therapies (Table 3.1). herefore, strategies to protect the brain af er trauma are based on an understanding of the pathophysiological processes of secondary brain damage.
Pathophysiological cascade
Energy failure
After the constant oxygen and glucose supply to the brain is interrupted, the electron transport chain within the mitochondria, and thereby oxidative phos-phorylation, is inhibited. Within 1-2 min this is followed by decreased levels of high-energy phosphates (phosphocreatine), within 4 min by depletion of glucose and glycogen stores, and after 5-7 min the cellular ATP concentration rapidly drops to near zero. his initiates several detrimental effects, such as the accumulation of lactic acid due to anaerobic glycolysis or failure of the energy-dependent transmembrane ion pumps.
Table 3.1 Consequences of cerebral oxygen and energy failure.
Permanent structural damage occurs within 5-15 min of oxygen deprivation, while hypoglycaemia can be tolerated for up to 60 min.
Tissue acidosis
During the initial minutes of cerebral ischaemia, brain metabolism switches from aerobic to anaerobic respiration, which rapidly produces lactic acid and acidifies the brain pH to 6.5-6.7. Hyperglycaemia aggravates this effect, leading to a tissue pH of 6.0. Acidosis itself might possess neuroprotective effects due to N-methyl-D-aspartate (NMDA) receptor blockade. However, in the presence of lactate presynaptic gluta-mate, reuptake is inhibited and excitotoxic neuronal damage is increased.
Membrane depolarization and cerebral oedema
A major consequence of the ATP shortage is a dysfunction of energy-dependent membrane ion pumps, allowing influx of Na+ and Ca2+ into the cell, and efflux of K+. High intracellular Na+ and Ca2+ concentrations are followed by CP and H. O influx into the neuron, leading to cytotoxic oedema and consecutively to an elevated intracranial pressure (ICP). Additionally, vasogenic brain oedem a, which is caused by mechanical or autodigestive disruption or functional breakdown of the endothelial cell layer (an essential structure of the blood-brain barrier) of brain vessels, increases the volume of the extracellular space. Disintegration of the cerebral vascular endothelial wall allows uncontrolled ion and protein transfer from the intravascular to the extracellular (interstitial) brain compartments, with consecutive water accumulation and a further increase in ICP.
Excitotoxicity and intracellular Ca2+ overload
The general energy depletion leads to a massive influx of Ca2+ into the cell and a failure of the energy-dependent presynaptic reuptake of excitatory amino acids. Both mechanisms increase the accumulation of excitotoxic neurotransmitter glutamate in the extracellular space and thereby maintain the excitotoxic cell damage. Hyperactivation of NMDA, a-amino-3-hydroxy-5-methyl-4-isoxazolpropionate (AMPA) and metabotropic glutamate receptors further increases intracellular Ca2+ and Na+ concentrations. Te intracellular Ca2+ overload appears to be one of the common pathways leading to irreversible cell damage and death. High intracellular Ca2+ concentrations trigger proteases that degrade cytoskeletal proteins (e.g. actin and spectrin) and extracellular matrix proteins (e.g. laminin). Furthermore, phospholipases A1, A2 and AC are activated, thus hydrolysing phospholipids within mitochondrial and cell membranes and generating free fatty acids (e.g. arachidonic acid). Tese can be metabolized to free radicals, prostaglandins and leukotrienes, which will change membrane permeability and ion distribution. Tese enzymes do not require oxygen or energy and therefore function during cerebral ischaemia .
Mitochondrial damage
The major purpose of oxidative phosphorylation in mitochondria is the continuous production of ATP by the oxidation of glucose. Ischaemic or traumatic challenges affect both inadequate delivery of oxygen and glucose, and impairment of mitochondrial function, leading to inadequate production of ATP. One of the key events that causes mitochondrial injury is an abnormal increase in intracellular Ca2+, which triggers the activation of degradative enzymes (e.g. calpain proteases and phospholipases) or the generation of enzymes that form reactive free oxygen radicals. Furthermore, high cellular Ca2+ levels activate the mitochondrial permeability transition where a non-selective, large conductance pore within the inner membrane opens, resulting in uncoupling of oxidative phosphorylation, osmotic swelling, release of matrix metabolites and even physical rupture of the mitochondrial outer membrane. Mitochondrial dysfunction leads to a loss of the mitochondrial membrane potential, a release of cytochrome c and apoptosis-inducing factor into the cytosol, and enhanced generation of free oxygen radicals, together promoting cell death .
Peri-infarct depolarization
Energy failure causes electrochemical and excitatory membrane depolarization of neurons and glial cells. his generates a wave of depolarization that moves away from the core lesion with a frequency up to eight events per hour. As repolarization is an energy-dependent process, which further stresses the metabol-ically compromised cells in the penumbra, peri-infarct depolarization and repolarization may contribute to the growth of the lesion.
Free oxygen radicals
Free oxygen radicals are physiologically produced in small amounts in cellular processes such as oxidative phosphorylation in the mitochondrial electron transport system. Under normal conditions, the generated superoxide radicals dismutate spontaneously or are enzymatically promoted (superoxide dismutase (SOD)) to hydrogen peroxide, which forms hydroxyl radicals. Hydroxyl radicals react with almost any intracellular molecule. Superoxide radicals can also react with nitric oxide (NO) creating the highly reactive peroxynitrite radical. he normal defence system against free radical damage includes enzymatic systems that quench or scavenge free radicals (e.g. SOD, catalase, glutathione peroxidase, and vitamins E and C). During cerebral ischaemia and reperfusion, the concentration of super-oxide and hydroxyl radicals increases tremendously and the normal defence systems are overwhelmed. As radicals can react with and damage virtually any cellular component, this excessive free oxygen radical overload further promotes the disintegration of the cell.
Inflammation
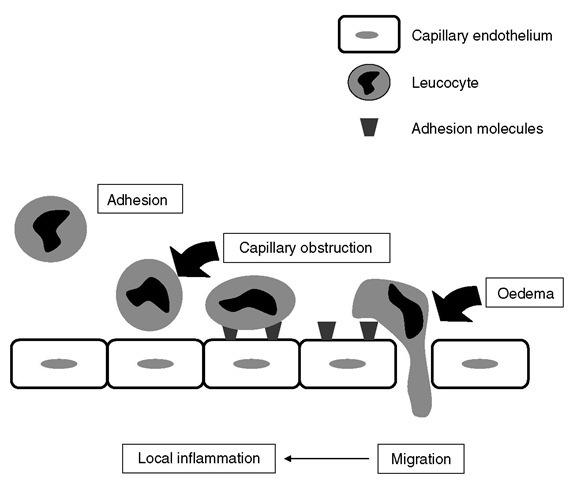
Brain injury induces a complex array of immunological/inflammatory tissue responses and it is still unknown how the different cellular and molecular components interact to contribute to inflammatory responses in the neuronal tissue. Both primary and secondary insults activate the release of cellular mediators including pro-inflammatory cytokines, prostagland-ins, free radicals and complement within hours. hese processes induce chemokines and adhesion molecules and in turn mobilize immune and glial cells in a parallel and synergistic fashion. For example, activated poly-morphonuclear leucocytes adhere to defective but also intact endothelial cell layers, as mediated through adhesion m olecules (Fig. 3.1). hese cells infiltrate inj ured tissue along with macrophages and T lymphocytes. Tissue infiltration of leucocytes is facilitated via upregulation of cellular adhesion molecules such as P-selectin, intercellular (ICAM-1) or vascular (VCAM-1) adhesion molecules, and integrins (CD11b/CD18). In response to these inflammatory processes, injured tissue will be eliminated, and within hours, days or weeks, astrocytes produce microfilaments and neutropines to ultimately synthesize scar tissue. Pro-inflammatory cytokines such as tumour necrosis factor (TNF), interleukin (IL)-1 p and IL-6 are upregulated within hours of injury. he progression of tissue damage relates to direct release of neurotoxic mediators or indirectly to the release of NO in toxic concentrations and cytokines. he additional release of vasoconstrictors (prostaglandins and leuko-trienes), the obliteration of microvasculature through adhesion of leucocytes and platelets, the blood-brain barrier lesion and the oedema formation further reduce tissue perfusion and – summa summarum – aggravate secondary brain damage.
Apoptoticand necrotic cell death
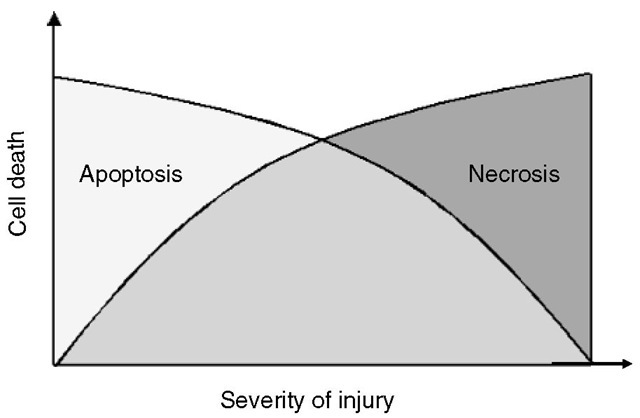
Two different types of cell death may occur following brain injury: necrosis and apoptosis (programmed cell death; Fig. 3.2). Necrosis takes place in response to severe mechanical or ischaemic/hypoxic tissue damage with excessive release of excitatory amino acid neu-rotransmitters and metabolic failure. Consecutively, phospholipases, proteases and lipid peroxidases aut-olyse biological membranes. Te resulting cell detritus is recognized as an ‘antigen’ and will be removed by inflammatory processes, leaving scar tissue behind.
Fig. 3.1. After neuronal damage,cellular and endothelial adhesion molecules are expressed facilitating the adhesion and penetration of immunocompetent cells such as leucocytes. This leads to cellular oedema and local inflammation (upregulation of pro-inflammatory cytokines).
Fig. 3.2. Apoptotic cell death occurs after minor injury of the cell and is the active energy-consuming form of cell death involving a ‘suicide programme’. The more severe an injury becomes, the less ATP is available and the more cells start to undergo necrotic cell death.
In contrast, neurons undergoing apoptosis, an active form of cell death involving a ‘suicide programme’, are morphologically intact during the immediate post-traumatic period with adequate ATP production providing a physiological membrane potential. Apoptosis is triggered by release of cytochrome c from the mitochondria into the cytoplasm (intrinsic pathway) or by activation of cell death receptors by their ligands (e.g. FasL or TNF-a; extrinsic pathway). Both pathways further mediate apoptotic cell death by the cleavage of different procaspases, which represent specific proteases of the IL-converting enzyme family. Te most recently detected apoptosis-inducing factor is also released from the mitochondria and activates apoptosis by a caspase-independent pathway. Apoptosis becomes evident hours or days after the primary insult: translocation of phosphatidylserine initiates discrete but progressive membrane disintegration along with lysis of nuclear membranes, chromatin condensation and DNA fragmentation; likewise, very small particles derived from condensed intracellular material (‘apoptotic bodies’) are removed from the shrinking cell by exocytotic mechanisms. Te nature of apoptosis generally requires energy supply and imbalance between naturally occurring pro-and anti-apoptotic proteins .
Experimental and clinical neuroprotection
Neuroprotection is defined as any strategy that antagonizes, interrupts or slows the sequence of injurious biochemical and molecular events that have the potency to cause irreversible cell death. In the past, more than 160 clinical trials have been performed investigating more than 50 different neuroprotective approaches (e.g. calcium-channel blockers, free radical scavengers and glutamate antagonists) that were able to improve neurological outcome in experimental models (Table 3.2). However, due to multiple reasons, none of these clinical trials was able to translate the experimental neuroprotective strategy to clinical settings.
Table 3.2 Investigated neuroprotective strategies
|
General strategies |
|
• Anti-excitotoxic interventions |
|
• Anti-inflammatory interventions (cerebral) |
|
• Protection against systemic infection |
|
• Antioxidants |
|
• Pre-/post-conditioning; remote conditioning |
|
• Anti-apoptotic interventions |
|
• Enhancing cerebral blood flow |
|
Specific drug therapy |
|
• Calcium-channel blocker |
|
• Anaesthetic agents |
|
• Glucocorticoids |
|
• Nitric oxide |
|
• Erythropoietin |
|
• Magnesium |
|
• Statins |