Laser Dopplerflowmetry
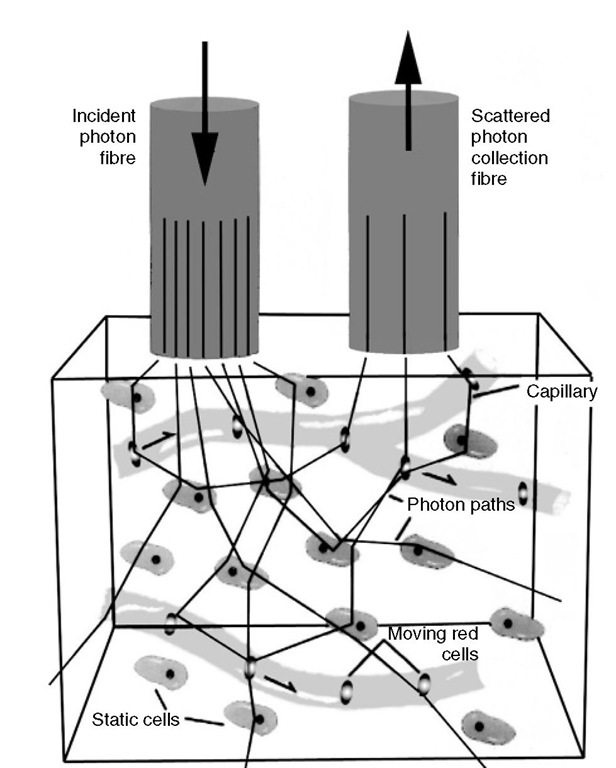
Laser Doppler flowmetry (LDF) is based on the assessment of Doppler shift of low-power laser light, which is scattered by moving red blood cells (RBCs) (Fig. 5.5).
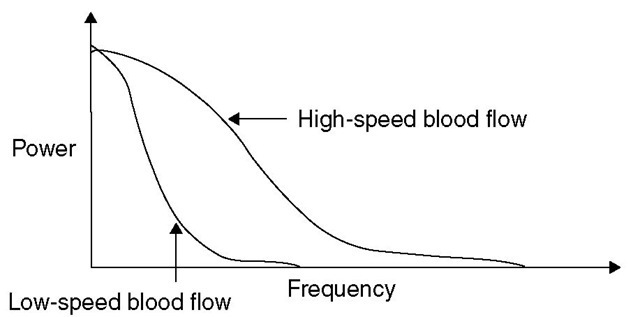
Briefly, monochromatic laser light, with a wavelength above the maximal absorption of haemoglobin and below the maximal absorption of water (600-780 nm), is delivered to and detected from a 1 mm3 volume of brain tissue by a flexible fibre-optic light guide. he laser light is scattered randomly by both static structures and moving tissue particles, mainly RBCs. Laser light reflected from stationary tissues remains unchanged in frequency, whereas light reflected by moving particles is both scattered and undergoes a frequency shift. Multiple scattering at various angles of incidence precludes the exact measurement of velocity of the moving RBCs; however, as the bandwidth of the Doppler shift frequencies increases linearly in proportion to the RBCs’ velocities, the mean frequency shift and the power are directly proportional to the velocity and the number of moving RBCs, respectively (Fig. 5.6).
Fig. 5.5. A graphic depiction of the principle of laser Doppler flowmetry.
Fig. 5.6. The theory behind laser Doppler flowmetry for the measurement of CBF. Doppler frequency and power depend on the speed of red blood cells. Bandwidth broadens as red blood cells speed increases, but amplitude and shape remain constant.
As the blood cell flux is equal to the velocity of the cells multiplied by their concentration, if the concentration of the RBCs remains constant, the power of the frequency-weighted Doppler spectrum is proportional to the RBC flux through the capillary bed and hence to CBF.
Laser Doppler flowmetry produces a continuous, real-time flow output, which is linearly related to CBF. Although laser Doppler flowmetry is a fast, continuous, non-radioactive bedside monitoring of CBF that can detect changes at the cellular level, there are still many practical as well as theoretical limitations to overcome. he device is invasive, requiring insertion of the probe during operation or via a burr hole. Changes in tissue perfusion are of en accompanied by changes in the tissue geometry and may affect flow measurements. Tissue density and geometry may also be altered af er brain injury, and therefore site selection is critical to the accuracy of the measurements. Probes measure flow within approximately 1.5 mm of the tips; hence, the measurement area is localized and caution must be exercised in making assumptions about global CBF. A further source of false readings is the presence of arterioles and venules, which amplify laser Doppler flowmetry signals, thereby over-representing microvascular blood flow.
Thermal clearance
Thermal diffusion flowmetry is used to estimate cortical blood flow by measuring changes in a temperature gradient, which exists between two thermistors within a probe applied to the cortex. he distal thermistor (active) measures flow via heat transfer to the capillaries while the proximal thermistor (passive) measures the baseline temperature. he primary measurement technique relies on detection of the temperature gradient between the large plate generating heat and the second smaller detector plate. he difference in temperature between the two plates is inversely proportional to the thermal conductivity of the brain tissue. he temperature gradient decreases as the flow increases so that:
where CBF is cerebral (cortical) blood flow, K is a constant, V is the voltage difference between the two plates at the time of measurement and V0 is the voltage difference at no flow.
Hie thermal diffusion CBF technique has many advantages in that it is simple, continuous and does not use ionizing radiation. Codman have produced an FDA-approved CBF monitor (Hemdex Cerebral Blood Flow Monitor) for continuous bedside measurement of CBF, measured in ml (100 g)-1 min-1. he monitor has been evaluated for use in aneurysm repair and extracranial/intracranial bypass surgery as a modality for intraoperative CBF monitoring.
Hie early results with thermal diffusion flowmetry have been promising for detection of vasospasm in SAH patients and for use as a bedside monitor for changes in carbon dioxide reactivity.
Limitations encountered with this technique include inaccuracies due to sudden temperature change (e.g. irrigation, rapid infusion of fluids) and motion artefacts. Meticulous probe placement is key, as the area monitored is localized to a sphere of tissue around 4-5 mm in diameter around the probe.
Transcranial Doppler ultrasonography
Transcranial Doppler ultrasonography (TCD) introduced by Aaslid and colleagues in 1982, is a noninvasive monitor that uses a Doppler transducer to measure RBC velocity and pulsatility of blood flow in the large vessels at the base of the brain.
As TCD measures RBC velocity and not flow, changes in flow velocity (FV) only represent true changes in CBF when both the angle of insona-tion and the diameter of the vessel insonated remain constant. Provided these limitations are recognized, the technique can be utilized to obtain information about CBF.
Principle of transcranial Doppler
Transcranial Doppler ultrasonography is based on the Doppler principle described in 1843 by Christian Doppler. he probe emits a wave with a known frequency in the order of2 MHz referred to as![]() , and propagation speed c towards the moving target, in this case RBCs. he echo perceived has an altered frequency,
, and propagation speed c towards the moving target, in this case RBCs. he echo perceived has an altered frequency,![]() which is dependent on the velocity of the RBCs. he difference between the
which is dependent on the velocity of the RBCs. he difference between the![tmpA2-84_thumb[2] tmpA2-84_thumb[2]](http://what-when-how.com/wp-content/uploads/2012/04/tmpA284_thumb2_thumb.jpg) is the Doppler shift,
is the Doppler shift,![]()
The reflected wave can be a higher or lower frequency, which is termed positive or negative shift.
Itie FV of the RBC is calculated as:
He FV determined by TCD is dependent on the cosine of the angle of insonation, represented as:
Hence, at 0° the TCD calculated and true velocity are equal (cosine of 0° = 1). However, at 90° the calculated
FV is 0, irrespective of the actual velocity. Fortunately, with the transtemporal window most commonly used to insonate anterior middle and posterior cerebral arteries, the anatomic limitations are such that signal capture is only possible at narrow angles (<30°) minimizing the error to <15%.
Itie other ultrasonic windows are transorbital for the carotid siphon and suboccipital for the basilar and vertebral arteries. It should be noted that up to 8% of the population does not have an adequate acoustic window.
Here is ample evidence suggesting that the diameter of the middle cerebral artery (MCA) does not change significantly with changes in arterial pressure, carbon dioxide partial pressure, or the use of anaesthetic or vasoactive agents. Hence, it is generally accepted that, during steady-state anaesthesia, changes in FV reflect corresponding changes in cortical CBF.
Practical application
Hie TCD system displays information as a velocity-time waveform. he peak systolic (PSV) and the end-diastolic (EDV) blood flow velocities are measured from the waveform display.
Calculated indices are described below.
Mean flow velocity
Hie range of normal values for adults was determined by Aaslid and colleagues.
Mean FV values >120 cm s-1 are generally considered abnormal. Both vasospasm and hyperaemia can lead to elevated FV. he Lindegaard ratio is an index used to distinguish between vasospasm and hyper-aemia by comparing the FV of the MCA with that of the extracranial portion of the ipsilateral internal carotid artery. he ratio of FV in the MCA to that in the external carotid artery increases with the severity of vasospasm. Hence, an FV ratio of <3 is consistent with hyperaemia, while 3-6 is indicative of mild vasospasm and >6 is consistent with severe vasospasm.
Waveform pulsatility
He pulsatility of the FV waveform reflects the resistance of the more distal cerebral vasculature. he most widely used measures are:
Fig. 5.7. Schematic representation of FV trace obtained from internal carotid artery bifurcation at 5.5-6.5 cm (a), the Ml segment of the middle cerebral artery at 3-6 cm (b), the posterior cerebral artery (Pl) at 6-8 cm (c) and the anterior cerebral artery (Al) at 6-8 cm (d). Flow above the horizontal is towards the probe, while flow below the horizontal is away from the probe.
where MV is mean velocity, and:
An increase in these indices has been correlated with an increase in ICP within the ICP range of 5-40 mmHg (Fig. 5.7).
Clinical applications of transcranial Doppler
Subarachnoid haemorrhage
Delayed ischaemic neurological deficit (DIND) secondary to vasospasm occurs in up to 30% of patients within 3-14 days after SAH. Early detection of clinically significant vasospasm can prompt the clinician to direct therapy towards prevention of long-term ischae-mic complications. Although correlations between TCD velocity and DIND has been reported by many authors, TCD velocity and CBF readings do not always correlate; moreover, intermittent TCD examinations may miss significant vasospasm. Daily TCD examinations may alert to the development of early vasospasm, especially in those sedated patients in whom assessment of neurology is not possible.
Intraoperative monitoring during carotid endarterectomy
Hypoperfusion and/or hyperperfusion may be responsible for a small number of perioperative strokes in patients undergoing carotid endarterec-tomy. Given that a TCD probe can be fixed without the interfering surgical field to provide a non-invasive continuous assessment of CBF, it is widely used for this procedure. Flow velocity MCA should be >40% of the preclamping value – a decrease below 15% can be used as an indication for shunt placement. Emboli are clearly audible during surgery, and waveform analysis can differentiate air from particulate emboli. Post-operatively, continuous TCD monitoring has been used to detect showers of emboli and patency problems with the graft. Furthermore, it is possible to assess cerebral autoregulation and the need for postoperative blood pressure control in order to avoid hyperaemic complications.
Traumatic brain injury
Transcranial Doppler ultrasonography can be used to provide a non-invasive assessment of ICP, cerebral autoregulation, carbon dioxide reactivity and the detection of vasospasm following TBI. Waveform pul-satility indices are known to correlate with ICP over a certain range, providing an estimation of distal cerebral resistance. he Lindegaard ratio helps differentiate vasospasm from hyperaemia, facilitating clinical management. A low FV of <35 cm s-1 has been correlated with poor neurological outcome.
In TBI, loss of cerebral autoregulation or carbon dioxide reactivity has been correlated with severity of injury, and may result in worse outcome and an increase in mortality.
Brain death
In countries where confirmation of death requires demonstration of CBF, angiography is considered to be the gold standard. here have been many case reports detailing the use of TCD to confirm cerebral circulatory arrest. As CPP approaches zero, three patterns of TCD waveforms are observed: oscillating flow, small systolic spikes and no signal. It is not clear which pattern is the most sensitive or specific for diagnosing cerebral circulatory arrest. Moreover, given that 8% of the population does not have an acoustic window, the results should be interpreted with caution.
In our opinion, TCD findings should not be used in isolation, as with any clinical measurement, but more to complement other monitoring modalities available in the neurointensive care .
![tmpA2-81_thumb[2] tmpA2-81_thumb[2]](http://what-when-how.com/wp-content/uploads/2012/04/tmpA281_thumb2_thumb.jpg)
![tmpA2-90_thumb[2] tmpA2-90_thumb[2]](http://what-when-how.com/wp-content/uploads/2012/04/tmpA290_thumb2_thumb.jpg)
![tmpA2-91_thumb[2] tmpA2-91_thumb[2]](http://what-when-how.com/wp-content/uploads/2012/04/tmpA291_thumb2_thumb.jpg)
![tmpA2-92_thumb[2] tmpA2-92_thumb[2]](http://what-when-how.com/wp-content/uploads/2012/04/tmpA292_thumb2_thumb.jpg)

![tmpA2-94_thumb[2] tmpA2-94_thumb[2]](http://what-when-how.com/wp-content/uploads/2012/04/tmpA294_thumb2_thumb.jpg)
![tmpA2-95_thumb[2] tmpA2-95_thumb[2]](http://what-when-how.com/wp-content/uploads/2012/04/tmpA295_thumb2_thumb.jpg)
