Schalley et al.[54] have ascertained that low yields are often obtained with these rotaxane ”snapping” syntheses because the nucleophilic anion required for rotaxane formation is often buried within the wheel and thus protected against attack of the semiaxle. To circumvent this problem, Ghosh et al.[55] have designed a centerpiece, which contains a phenolate group and two sites for stopper attachment. Complete deprotonation of the phenolate using Schweisinger’s Pj base forms a supra-molecular complex with the axle bound within the wheel. The final step is the attachment of the stopper units via amide bond formation to yield [2]rotaxanes in 20-30% yield (Scheme 3). Rotaxane formation is confirmed by both 1H NMR and matrix-assisted laser desorption initiation (MALDI) mass spectrometry.
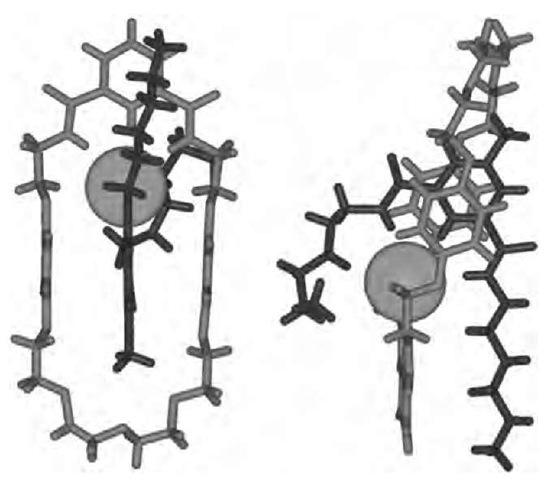
Fig. 10 Stick representation of the pseudorotaxane crystal structure. Left: View through the annulus of the macrocyclic ring. Right: View in the plane of the macrocyclic ring. Chloride represented as CPK sphere for clarity.
However, computer modelling results suggest that a phenolate-wheel complex can form in which the components are not threaded, with both arms of the axle on the same side. This is primarily because of the flexibility of the centerpiece; therefore the addition of stoppers can result in a noninterlocked axle-wheel complex. Increased rigidity in the axle may lead to higher rotaxane yields. The functionalized centerpiece raises the possibility of inducing molecular motion within related systems, especially if phenol-to-phenolate interconversion can easily be achieved.[55]
Fig. 9 The chloride anion templates the self-assembly of the threadlike and macrocyclic components giving an interlocked structure.
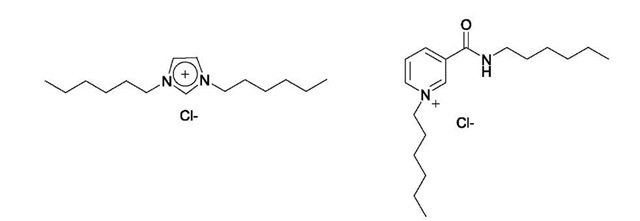
Fig. 11 Imidazolium (left) and nicotinamide (right) threads used by Beer et al. in the assembly of pseudorotaxanes.
Recent reports from Li et al.[56] have been concerned with the anion-templated synthesis of related rotaxanes using modified tetralactam wheels that have exo-oriented bipyridine functionalities capable of complex-ing metal ions.
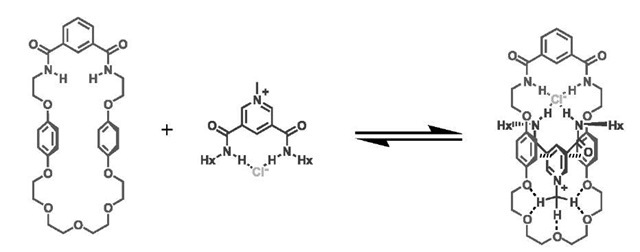
Beer et al. have since begun exploiting anions in the templated synthesis of pseudorotaxanes and rotaxanes. Inspired by the demonstration of anion recognition by simple isophthalamide molecules by Kavallieratos et al.[57,58] they have designed a novel assembling motif consisting of two amide clefts orthogonally disposed about a central chloride anion core. Neutral isophthala-mide receptors bind chloride in 1:1 stoichiometry and so are unsuitable for assembly purposes. However, the use of a cationic molecule as one of the ligands enables two receptors to bind to the halide anion.[59] As a consequence of increased amide acidity because of the cationic nature of the pyridinium ring, the chloride counter-anion is bound much more tightly compared with an analogous isophthalamide neutral compound. The tight ion pair leaves the chloride anion coordinatively unsaturated in noncompetitive solvents, with an empty meridian exposed, which is capable of coordinating with another suitable hydrogen bonding ligand. Incorporation of a neutral isophthalamide fragment into a macrocyclic structure provides the wheel component of the pseudor-otaxane through which the cationic component can be threaded in noncompetitive solvents. The general strategy adopted by Beer et al. is illustrated in Fig. 8.
For the pyridinium pseudorotaxanes first reported, additional stability is provided by p-p interactions and hydrogen bonding (Fig. 9).[59] A high degree of selectivity was exhibited in these systems, with the pyridinium threads binding in the order Cl->Br->I->PF-. Therefore the system not only self-assembles but also displays a high degree of anion-specific recognition.
The crystal structure of the pyridinium-based pseudo-rotaxane reveals the threaded nature of the components (Fig. 10) and provides evidence of p-p acceptor-donor interactions and hydrogen bonding between the pyridi-nium methyl group and the polyether chain.
Further work has demonstrated the versatility of this assembling motif by the formation of pseudorotaxanes based on nicotinamide and imidazolium cationic threads (Fig. 11).[60] Neither of the threads have hydrogen bond donors capable of binding to the polyether ring and although the former has one amide group capable of anion binding, the latter has none and threads simply by virtue of being an ion pair.[60] The chloride anion can be seen to ”drag” the cationic thread into the macrocycle, and further stabilization is provided by second-sphere acceptor-donor interactions. With nico-tinamide and imidazolium, selective macrocycle binding for chloride threads over hexafluorophosphate threads was demonstrated.
Scheme 4 Beer et al.’s anion-templated rotaxane synthesis.
Fig. 12 Crystal structure of Beer et al.’s [2]rotaxane. (a) Stick representation of the solid-state structure illustrating the interlocked nature of the components (solvent is omitted and only one occupancy of the tert-butyl groups is shown). (b) Solid-state structure of the [2]rotaxane showing the enclosed cavity. Chloride anion shown as CPK for clarity.
Wisner et al.[61] have also reported on the extension of this work to the anion-templated formation of [2]rotaxane. An acyclic chloride anion template based on simple pyridinium pseudorotaxane threads has been designed, which acts as the ”axle” of the rotaxane. The second component is a neutral acyclic molecule incorporating an isophthaloyl anion binding cleft and has two long side chains terminating in allyl groups capable of ring closing metathesis (RCM). The side chains each possesses a hydroquinone group, as with the original pseudorotaxane macrocycle (to facilitate donor-acceptor interaction), and polyether chains capable of hydrogen bonding. The two components associate strongly in noncompetitive solvents, and rotaxane formation facilitates ring closing metathesis of the neutral component about the ion pair using Grubbs’ catalyst with yields up to 47% obtained (Scheme 4).[61] No rotaxane formation occurs with pyridinium bromide or hexafluoro-phosphate salts, indicating the templating nature of the chloride anion.
Fig. 13 Molecular structure of a Kruger’s double helicate.
An impressive feature of this method of rotaxane assembly is that the resultant product retains a degree of functionality based on the anion template itself. Exchange of the templating chloride anion for the noncompetitive hexafluorophosphate anion leaves a highly selective binding site within the rotaxane. Binding studies have shown that although the pyridinium PF- thread alone binds anions with a selectivity trend AcO- ^ H2PO- >Cl-, the pyridinium PF- rotaxane exhibits a complete selectivity reversal and a high selectivity for chloride (i.e., the templating anion). Beer et al. assign this binding selectivity to the creation of a unique hydrogen bond donating pocket within the [2]rotaxane superstructure, which possesses a complementary topology to chloride (Fig. 12). Larger anions cannot penetrate this diamide cleft and bind at the periphery of the rotaxane, or via a large displacement of the pyridinium thread from the macrocyclic cavity. Therefore this example demonstrates not only anion tem-plation for the synthesis of mechanically interlocked components but also shows how a rigorous design procedure has led, via self-assembly, to a functional supra-molecule whose properties are dependent on the templation process.
Fig. 14 Guanidinium-appended porphyrins (R = H, SiPh2But).
Fig. 15 Hydrogen bonding between 2,5-diamidopyrrole anions (R=Ph).
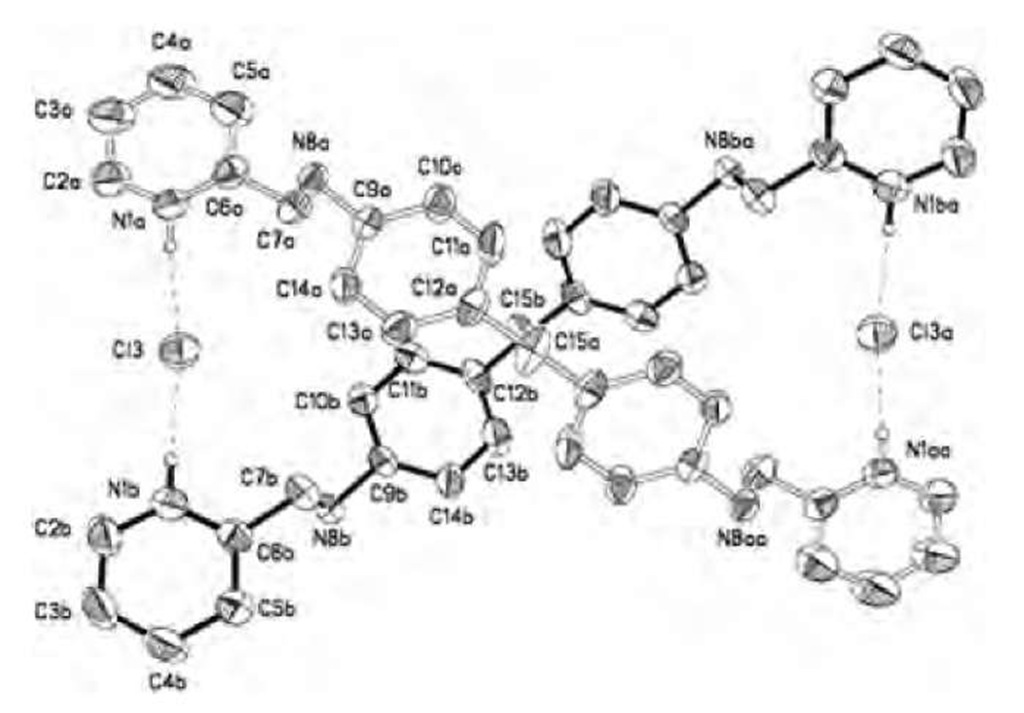
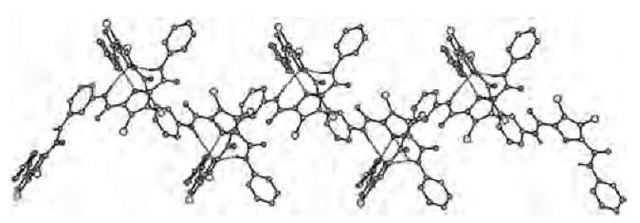
Keegan et al.[62] have reported the first structurally characterized anion-directed assembly of a dinuclear double helicate. The di-ammonium-bis-pyridinium salt [(H4LCl)2] • 6HCl • H2O, where L is a diamino-bis-pyri-dine ligand, assembles into a double helical structure, featuring a major and a minor groove, with two ligands wrapping around two chloride anions (Fig. 13). The chloride anions are coordinated by two pyridinium moieties and interact weakly with methylene and aromatic CH groups.
Camiolo et al.[63] have demonstrated the anion-con-trolled assembly of porphyrin-bicyclic guanidinium conjugates in aqueous solution. Exploiting the cooperative interaction of porphyrin and guanidinium moieties with anions, they have reported the assembly of structures in which chirality is controlled by the anion template used (Fig. 14).
Fig. 16 Gale’s diamidopyrrole ligand.
Fig. 17 X-ray crystal structure of Gale’s ”anion-anion” assembled solid-state polymer.
Detailed investigations using, primarily, circular di-chroism indicate that the assembly process is achieved by the presence of small achiral anions. The authors propose that the anions serve as linkers that diminish repulsive forces between the porphyrin units.
Fig. 18 Crystal structure of Gale’s fluoride templated helix. Top: Side view. Bottom: Top view showing p-stacking interactions. TBA salts and some hydrogens omitted.
Gale et al. have been investigating the use of pyrroles in anion-templated self-assembly. In 2002, they reported on the deprotonation and subsequent dimerization of a series of simple 2,5-diamidopyrrole compounds. It was shown that the anionic diamidopyrrolate ion can recognize another diamidopyrrolate ion through hydrogen bonding.[64]
The components arrange themselves orthogonally to each other (Fig. 15). Not only is NH.. .N~(pyrrole) hydrogen bonding observed in this case, but p-H interactions are also present.[64]
The first extension of this work was the so-called ”anion-anion” assembly of a supramolecular polymer.1-65-1 These polymers are based on two pyrrole units linked together and are the first example of interlocked materials based on hydrogen bonding where both components are anionic. One of the ligands developed is shown in Fig. 16.
Crystallization of this ligand from acetonitrile in the presence of excess TBA fluoride gave the doubly de-protonated anions forming interlocked chains of anions via NH.. .N_ hydrogen bonding (Fig. 17).[65]
Coles et al.[66] have also extended their self-assembly interests to the use of isophthalamide clefts in the formation of a double helix. This is the first reported use of neutral ligands forming anion-templated helicates. A ligand is designed featuring an anion binding amide cleft and electron-withdrawing groups to enhance ligand-anion interactions. A crystal structure of the ligand in the presence of fluoride anions was obtained and shows the double helix structure formed around two fluoride anions, again via N-H… F hydrogen bonds (Fig. 18). Further stabilization is provided by p-p interactions between terminal nitroaromatic groups.
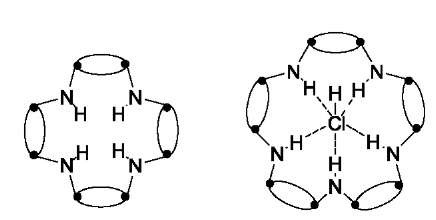
Bashall et al.[67] have recently reported the synthesis of a series of macrocycles containing [{P(m-NtBu)2}(m-NH)]n frameworks. A tetrameric macrocycle is formed on the reaction of [ClP(m-NtBu)]2 with [NH2P(m-NtBu)]2 in the presence of a base (THF/Net3). However, addition of an excess of LiCl results in the formation of a pentameric macrocycle [{P(m-NtBu)2}(m-NH)]5(HCl), with the ratio of tetrameric to pentameric being influenced strongly by excess chloride (Fig. 19).
Fig. 19 Wright’s tetrameric macrocycle (left) and chloride anion-templated macrocycle (right).
Structural characterization of the pentamer indicates that the chloride anion is positioned at the center of the macrocycle and is hydrogen-bonded to the five NH groups of the ring.
CONCLUSION
This review is intended to provide a general discussion of the emerging field of anion-templated self-assembly in organic systems. It can be seen that the range of organic compounds self-assembled by anion templates is highly diverse. Rational design has been employed from the start, and the field has rapidly developed to the stage at which topologically complex structures, such as rotaxanes, are being prepared.
As with self-assembly as a whole, the range of complex molecules prepared by self-assembly techniques, which would otherwise be practically impossible using covalent synthesis, is becoming increasingly diverse. A bright future for anion-templated self-assembly seems highly likely, with the above results illustrating the rapid progress made in such a short time span. There is no doubt that even on a short-term basis, many more impressive results will be seen within the literature and it remains the authors’ view that anions will have a key role to play in the future of self-assembly.

![Crystal structure of Beer et al.'s [2]rotaxane. (a) Stick representation of the solid-state structure illustrating the interlocked nature of the components (solvent is omitted and only one occupancy of the tert-butyl groups is shown). (b) Solid-state structure of the [2]rotaxane showing the enclosed cavity. Chloride anion shown as CPK for clarity. Crystal structure of Beer et al.'s [2]rotaxane. (a) Stick representation of the solid-state structure illustrating the interlocked nature of the components (solvent is omitted and only one occupancy of the tert-butyl groups is shown). (b) Solid-state structure of the [2]rotaxane showing the enclosed cavity. Chloride anion shown as CPK for clarity.](http://what-when-how.com/wp-content/uploads/2011/03/tmpD5163_thumb_thumb.jpg)