ALKYLSILANE SAMS ON SILICA
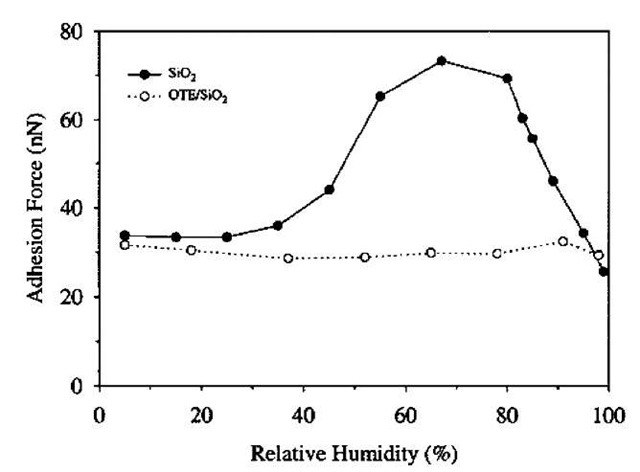
The effect of humidity on the adhesion of alkylsilane SAMs deposited on Si/SiO2 surfaces has been more thoroughly investigated compared with alkylthiols on gold in part because of the importance of antiadhesive coatings in silicon-based MEMS devices. An important difference between SAMs formed by alkylthiols and organosilanes is the possibility for the formation of cross-linked siloxane bridges (Si-O-Si) between adjacent molecules. It is commonly assumed that this cross-linking stabilizes these SAMs.[26] However, it is not clear as to what extent this actually occurs because the cross-linking should be sterically hindered.[27] Zhang et al.[28] investigated the effect of humidity (up to 80%) on the adhesive force between an AFM silicon nitride tip and organosilane SAMs with different terminal groups (-CH3, -COOCH3, and -COOH) deposited on hydrogen-terminated silica Si(111). Adhesion to hydrophilic SAMs increased at the highest humidities because of capillary condensation, whereas there was no change in the adhesive force between the tip and the methyl-terminated SAM. He et al.[29] also found that the adhesive force between an AFM tip rendered hydrophobic by coating with n-octadecyltrichlorosilane (OTS) and a silicon surface did not change for humidities up to 80%. Xiao and Qian[15] examined the adhesion between an AFM silicon nitride tip and an n-octadecyltriethoxysilane (OTE)-coated silicon surface, and found essentially no dependence on humidity up to 98%, as shown in Fig. 4. Interestingly, adhesion between the tip and a bare hydrophilic silica surface first increases with increasing humidity because of capillary condensation and then decreases close to saturation. Because the tip radius is small, the filling angle becomes large near saturation. According to Eq. 6, the effect of an increasing filling angle is a decreasing adhesive force, as is observed. The net effect is that the adhesive forces on the bare silica surface and the OTE-coated silicon surface are the same near saturation. For practical purposes, the AFM experiments discussed here show that the hydrophobic SAM affords protection against capillary-induced adhesion even at high humidity.
Fig. 4 Measured adhesion force as a function of humidity for SiO2 and OTE/SiO2 against a Si3N4 AFM tip.
With a specific focus on MEMS, some workers have developed an in situ microcantilever beam technique for measuring adhesion using an as-fabricated MEMS test structure constructed from polycrystalline silicon (polysilicon) that exhibits nanometer-scale rms rough-ness.[30-32] Capillary condensation complicates the fabrication as well as the operation of MEMS, so much effort is being directed at antiadhesive strategies. Hydrophobic SAMs are being investigated as coatings for MEMS devices as these coatings reduce adhesion. De Boer et al.[30] studied the effect of humidity on the adhesion of perfluorotrichlorosilane (FDTS)-coated (from solution in isooctane) cantilever beams to an FDTS-coated substrate. For humidities up to 80%, adhesion of the beams was not observed to change significantly. After a 4-hr exposure at 90% RH, a mild adhesion increase was observed, and a much more significant increase was observed after 7 hr of exposure (Fig. 5). AFM topographic images showed mounds, strongly suggesting that increased adhesion correlates with this structural change of the surface. The authors hypothesized that the mounds are the result of humidity-induced restructuring from a surface monolayer phase to a lyotropic bulk phase. Mayer et al.[32] coated cantilever beams with monolayer films of trideca-fluoro-1,1,2,2-tetrahydrooctyltrichlorosilane (FOTS) in a chemical vapor deposition process. These coatings are hydrophobic, and adhesion tests on coated and uncoated MEMS test structures demonstrate superior performance of FOTS coatings. Cleaned, uncoated, cantilever beam structures exhibit high adhesion energies in a high-humidity environment (100 mJ/m2 for >90% RH) because of capillary condensation. FOTS-coated beams exhibit negligible adhesion at low humidity and < 20 mJ/ m2 adhesion energy at >90% RH. No obvious film degradation was observed for films exposed to >90% RH at room temperature for ~ 24 hr. The authors suggest that chemical vapor deposition of FOTS affords greater stability in high-humidity environments compared with solution-based deposition.
Fig. 5 Interferograms of adhesion hysteresis observed at RH=90% after a 7-hr exposure. The images are compressed by a factor of three in the horizontal direction relative to the vertical. Twenty-five beams run horizontally, are 10 mm wide, and are spaced 5 mm apart. Their lengths are from 1500 mm (bottom of each image) to 2000 mm (top). Beams longer than 1700 mm are cut off because of the charge-coupled device (CCD) camera field limit. The voltage was increased up to 160 V before decreasing.
Patton et al.[4] evaluated the effect of an OTS SAM coating on the tribological performance of a MEMS electrostatic lateral output motor. For uncoated motors, excessive wear of sliding contacts and permanent adhesion of static contacts were observed at 0.1% RH. Degradation of electrostatic force and high stiction forces limited dynamic performance and reliability, and caused device sticking at and above 70% RH because of capillary condensation. Around 50% RH, uncoated motors exhibited negligible wear, low adhesion, and a wear life at least three orders of magnitude longer than in dry conditions. Water vapor behaved as a gas-replenishable lubricant. The OTS coating broadened the operating envelope to 30-50% RH and reduced stiction, which allowed better dynamic performance at high RH. At high RH, stiction problems recurred when the OTS coating was worn away. By controlling and optimizing the amount of adsorbed water with the OTS coating, excellent performance, low friction and wear, and excellent durability were attained with the lateral output motor.
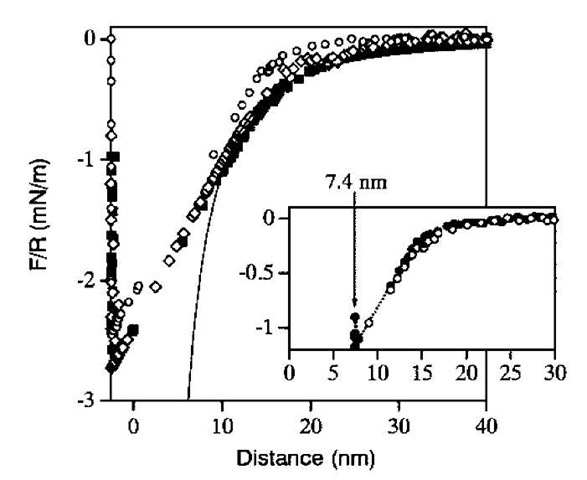
It is generally thought that organosilane SAMs cova-lently bind to silica, imparting enhanced stability.[33] However, infrared spectroscopic studies indicate that few, if any, bonds are formed and that with exposure to water over time, siloxane bonds hydrolyze.[34] It is difficult to determine how water vapor interacts with the SAM on the molecular level from the AFM and cantilever beam studies. Ohnishi et al.[35] measured the force between heptadecafluoro-1,1,2,2,-tetrahydrodecyltriethoxysilane (FTE) SAMs deposited on identical fused glass surfaces (radius of curvature ~ 2 mm) using the interfacial gauge.[36] The force as a function of surface separation was measured in dry conditions, near saturation, and in water. In humid air, the force curve obtained as the surfaces were brought together was shifted toward larger distances compared with dry conditions, presumably because layers of water were adsorbed between the substrate and the fluorocarbon layer (Fig. 6). No capillary condensation forces were detected with increasing humidity even though SAM thickness increased.[37] This suggests that in this case, the silane SAM is not covalently bound to the silica surface. The response to humidity is reversible in that if the water vapor is removed, the forces measured in dry conditions are recovered.
Fig. 6 Measured forces on approach to saturated water vapor of fluorinated surfaces prepared by three different methods: (o) Langmuir-Blodgett deposition; (o) FTE adsorbed from CHCl3; (■) FTE adsorbed from the neat solution. In the inset of the lower figure, the force profile of Langmuir-Blodgett in humid air (shifted to larger distances) is compared with that in dry air (……•……). The solid line is a fit obtained with the equation for the capillary force (Eq. 4).
ALKYLSILANE SAMS ON MICA
Alkylsilanes have attracted attention because they are capable of cross-polymerization and covalent attachment to SiO2-based oxide surfaces such as oxidized silicon wafers. Mica also contains Si-O bonds, but there are no inherent functional groups on the surface to serve as attachment sites. SAMs on mica are potentially useful in nonlinear optics because mica can be prepared as relatively large, molecularly smooth sheets.[1]
Several research groups have investigated the deposition of alkylsilanes, mostly OTS and OTE, on mica.[38-12 These monolayers are hydrophobic as judged by the contact angle of water, but in some cases, the contact angle decreases with prolonged exposure to water.[42,43] Tian et al.[39] investigated the effect of humidity on the friction force on OTE SAMs on mica. On bare mica, water acts as a lubricant and the friction force decreases gradually as humidity increases. At low humidities, an OTE SAM coating also serves as a good lubricant, reducing friction. As humidity increases, friction increases, indicating that water in contact with the OTE SAM decreases its lubricating effectiveness. Above ~ 30% RH, the film breaks down at a critical load and the tip contacts bare mica. The load required to cause breakdown decreases as humidity increases, indicating that the presence of water makes it easier to remove the SAM. Because the OTE SAM is not fully compact because of long-range disordering, condensed water can penetrate through the film to the OTE-mica interface. The accumulation of water at the interface weakens the OTE-mica links as well as the links between OTE molecules; therefore the film quality deteriorates.
Kim et al.[42] used the SFA to study OTE SAMs on mica. In this case, the OTE SAM was deposited on both bare mica and plasma-pretreated mica. Because mica has no functional groups to which the OTE can bond covalently, plasma treatment was used to break surface Si-O-Si bonds to create an opportunity for covalent attachment between the OTE SAM and mica. In this work, OTE SAMs deposited on both untreated and plasma-treated mica were initially highly hydrophobic, but contact angle hysteresis indicated that water interacts favorably with the monolayers on prolonged exposure. Defects in the monolayer most likely make it possible for the water to reach the hydrophilic region between the silane headgroups and the mica. More water was adsorbed by the monolayers on untreated mica compared with the plasma-treated case. This work strongly indicated that if the SAM is not firmly anchored to the substrate, water can accumulate in the region between the SAM and the substrate. Near saturation, water droplets also condensed on the surface presumably as monolayer defects regardless of how well the SAM was anchored. At very high humidities, a capillary condensed annulus of water was observed around the contact zone.
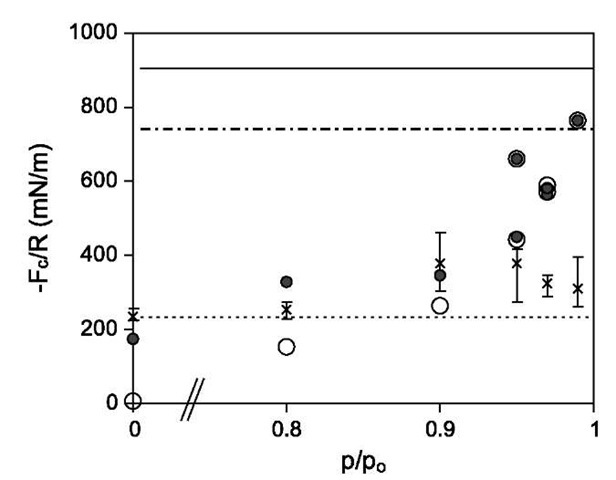
In a further study, Kim et al.[44] used the SFA to investigate the effect of humidity on surface deformation and adhesion of untreated and plasma-treated mica surfaces coated with OTE SAMs. In general, when two solid bodies come into contact, they deform in a way that is determined by their elastic or viscoelastic properties, by the surface forces between them, and by any externally applied load.[45] Because of the practical importance of understanding interparticle adhesion, the relationship between surface deformation and adhesion has been studied extensively.[18] Capillary condensation acts outside the contact area and modifies the adhesive force in two interrelated ways: first, by altering the surface deformation; and, second, by increasing the adhesive force because of the Laplace pressure in the annulus. In the presence of a capillary condensed liquid bridging the two surfaces, the measured adhesive force has contributions both from the direct solid-solid adhesion and from the Laplace pressure arising from the liquid-vapor interface.[46] In this study, the size of the contact area was measured as a function of applied load and the contact mechanics-based Maugis model outlined in Eqs. 12-14 was applied to extract the capillary condensation and solid-solid contributions to the adhesive force. The SFA is particularly well suited for these experiments because the interferometric technique allows direct observation of the surface shape and size of the contact zone. Pulloff forces were also measured by pulling the surfaces apart. Fig. 7 shows adhesive forces obtained from both methods. From load-vs.-contact area measurements, it is clear that the adhesive force because of capillary condensation depends on humidity. It decreases from the highest values near saturation to zero in dry conditions. This is not unexpected and has been shown previously.[47] However, Eq. 5 predicts that the adhesive force because of capillary condensation is independent of humidity. There is agreement with Eq. 5 at high humidities. At low humidities before capillary condensation is detectable, pulloff forces agree well with predicted adhesive forces determined from force-vs.-contact area measurements and the Maugis model. At high humidity, force-vs.-contact area values are generally higher than the pulloff force measurements.
Fig. 7 Normalized adhesive force for OTE on untreated mica as a function of RH. The open and closed circles denote the Laplace pressure contribution to the adhesive force and the total adhesive force predicted from the Maugis model. Experimental pulloff force measurements are denoted by x symbols, with error bars indicating the range of measured values. Horizontal lines denote the normalized adhesive force due to capillary condensation as calculated from Eq. 5 with 81 = 82 = 0° (—), 35° (—), and 75° (—).
It is suggested that the difference is because of the history of the surfaces prior to pulloff. In force-vs.-area measurements, a load is applied to push the surfaces together before pulling them apart slowly, whereas in pulloff force measurements, the surfaces are separated more rapidly without applying an extra load. This work suggests that adhesion between OTE SAMs on mica is not dominated by capillary condensation below ~ 80% RH. At higher RH, the effect of capillary condensation on the adhesive force is significant and depends on the amount of compression prior to detachment.
CONCLUSION
Cappilary condensation increases adhesion between contacting surfaces in humid environments. Hydrophobic SAMs reduce this effect by reducing surface wettability. SAMs must be resistant to water-induced degradation to effectively reduce adhesion in the long term. There are relatively few studies that address the effect of humidity on SAMs despite the importance of this effect in practical applications. Among these studies, there is substantial variability in the reported effectiveness of hydrophobic SAMs in reducing adhesion in humid environments. This depends on the particular SAM, the substrate, and the SAM deposition method. Even for initially hydro-phobic SAMs, exposure to humidity over long time periods is shown to reduce SAM stability and effectiveness in some cases. Further work in this area is needed to fully understand the effect of humidity on SAM stability and adhesion.