INTRODUCTION
Buckminsterfullerene C60 was first detected in the time-of-flight mass spectrometer from the product of laser vaporization of graphite[1] and later obtained in large quantity from electric arc resistive heating of graphite rods.[2,3] The four characteristic peaks in the infrared (IR) spectrum of C60 revealed its truncated icosahedron structure,1-3-1 which was further confirmed by nuclear magnetic resonance (NMR) spectroscopy.1-4-6-1 Separation and characterization of fullerenes C70,[4,5,7] C76,[8] C78,[9-12] C82,[11] and those with even higher carbon atom counts followed. High-performance liquid chromatography (HPLC) has proved to be a powerful technique to separate fullerene isomers, while 13C NMR is the method of choice to characterize them.
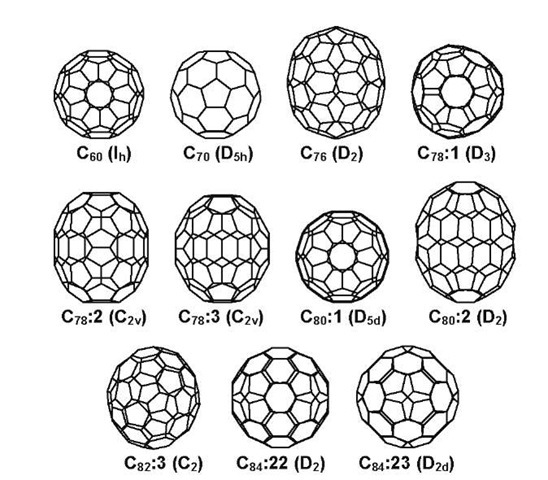
The cage-like structure of a fullerene consists of 12 five-membered rings and a number of six-membered rings depending on the number of carbon atoms.[13] It was postulated that the five-membered rings need to be separated from each other to reduce the localization of the strain caused by the bending of the sp2-hybridized carbon atoms. This is the essence of the isolated pentagon rule (IPR),[13] which dramatically reduces the possible number of isomers for a given fullerene family. Fig. 1 shows the major isomers of fullerenes C60 to C84. On the other hand, it is still common that several IPR isomers of a fullerene have same point-group symmetry and have same 13C NMR patterns. For instance, isomers 1, 5, 21, and 22 of fullerene C84 all have D2 symmetry and give 21 NMR peaks with equal intensity. One of the two major isomers of C84 has D2 symmetry as observed in experiment, but its structure cannot be determined from the one-dimensional (1-D) NMR spectrum.1-11,12-1 Relative energies calculated using quantum mechanics are helpful, but not definitive in determination of the molecular structure. Accurate theoretical chemical shifts predicted by high-level quantum mechanical calculations have been shown to result in unambiguous assignment for those isomers.1-14,15-1
This entry reviews the identification of the fullerene isomers based on theoretical 13C NMR chemical shifts. All the studied isomers have been separated by HPLC and characterized by NMR spectroscopy, and their NMR chemical shifts are taken from literature. We first briefly review the experimental aspects of the generation, separation, and characterization of fullerene isomers and earlier theoretical attempts to assign these isomers. Subsequently, the predicted NMR spectra for nine isomers of fullerenes C70, C76, C78, C80, and C84 are compared with experimental data, demonstrating the accuracy of the density functional theory (DFT) calculated chemical shifts and, at the same time, confirming the experimental structural assignment. The discrepancy between experiment and theory in the NMR spectra of the D5d isomer of fullerene C80 is discussed. Then the predicted NMR spectra for fullerenes C82 to C90 are summarized, and the identification of 11 previously unassigned isomers is achieved for which good experimental spectra are available. The reported results on theoretical NMR chemical shifts for charged and substituted fullerenes are also briefly reviewed. Finally, the relationship between chemical shift and local connectivity, and the energetics of the stable fullerene isomers are discussed.
EXPERIMENTAL ISSUES
Fullerenes were first detected in the product of laser vaporization of graphite.[1- Although this method gave sufficient amounts of fullerenes for mass spectroscopic measurement, it is not suitable to produce samples large enough for separation. Gram-scale samples of fullerenes were obtained by resistive heating,[2,3- electric arc dis-charge,[16] and plasma discharge of graphite.[17] Fullerenes up to C108 have also been obtained from the condensable material from flat premixed benzene/oxygen/ argon flames.[18,19] Recently, the rational chemical synthesis of C60 has been achieved by laser irradiation[20- of polycyclic aromatic hydrocarbon C60H30 and later in isolable quantities by flash vacuum pyrolysis[21- of C60H27Cl3 at 1100°C. Because no other fullerenes were produced as byproducts, this method raised the possibility of producing fullerenes by target-specific synthesis.
Fig. 1 The major isomers of fullerenes C60, C70, C76, C78, C80, C82, and C84.
The resulting fullerenes are normally first extracted using various organic solvents, then separated by HPLC. Characterization of the fullerenes includes mass spectrum, UV-Vis, IR, and NMR measurements. Among the characterization techniques, 1-D 13C NMR spectrum is easily obtained and directly related to the molecular structure, thus commonly used for fullerenes. The 3He NMR spectra have also been used to distinguish among isomers as well as to probe the magnetic shielding environment inside the fullerene cavity.[22,23] We will focus in this entry on the identification of fullerene isomers based on 1-D NMR spectra.
EARLIER THEORETICAL ATTEMPTS
Much attention was focused on the energetic aspects of fullerenes, while considerably less effort has been paid to their magnetic properties. The magnetic susceptibility of buckminsterfullerene C60 was studied in the early theoretical studies[24-26] before the fullerene samples were obtained. After fullerene samples became available, the prediction of NMR chemical shifts has been an active subject to identify the observed isomers. Fowler and Manolopoulos[13]27] presented all mathematically possible structures for fullerenes C60-C100 and compiled the structures for all IPR-abiding isomers. Haser et al.[28] used the gauge-independent atomic orbital-coupled perturbed Hartree-Fock (GIAO-CPHF/DZP) method to calculate the chemical shifts of C60 and C70. In the first attempt to identify the observed D2 isomer of C84, Schneider et al.[29] calculated the NMR spectra for isomers 5, 22, and 23 (isomer numbering after Fowler and Manolopoulos, which will be followed here) using the GIAO-SCF/TZP level of theory. They concluded that isomer 22 was the most likely structure because the spectral span of isomer 5 was much larger than that of the observed isomer and isomer 22 was favored over isomer 21 on energetic grounds. The calculated NMR spectral spans were used to rule out the D2(Ih) isomer of C80 as the experimentally observed D2 isomer.[30] Heine et al.[31] used the individual gauge for local orbitals-density functional based tight binding (IGLO-DFTB) method to study fullerenes C70, C76, C78, five isomers of C84, (C36)2, and (C6o)2. Qualitative agreement with experiment was achieved for the experimentally distinguished isomers, and assignment of the D2 isomer as 22 was supported. A full survey of the 24 isomers of C84 was later reported.[32] The structure of C70 was solved by Hedberg et al.[33] in gas-phase electron diffraction experiments with the help of NMR chemical shifts calculated at the GIAO-SCF/TZP level of theory. Accurate chemical shifts of C60 and C70 were calculated using Hartree-Fock (HF) and DFT methods by Buhl et al.[34]
COMPUTATIONAL METHOD
While the relative energies of the fullerene isomers calculated from total electronic energies by various quantum mechanical methods play an important role in determining the structures of the observed isomers, it is not definitive because the observed isomers have to survive the high temperature used in the production process. Direct comparison between accurate theoretical chemical shifts and experimental values is necessary to assign the observed fullerenes as NMR is the commonly used characterization technique and chemical shift directly relates to the chemical environment of the nucleus. In early 1998, it appeared to us that the calculation of NMR chemical shifts for molecules of the size of fullerenes became practical using ab initio or DFT methods with medium basis sets. Because the DFT method[35] in the form of B3LYP[36] hybrid functional had been shown to give better geometries and vibrational frequencies for a wide range of molecules than ab initio HF method, it was tested to predict the NMR chemical shifts of isomers 22 and 23 of fullerene C84.[15] The results were in very good agreement with experimental data and led to the prediction of NMR chemical shifts for other fullerenes and subsequent assignment of their isomers. The calculation of NMR properties of fullerenes has recently been reviewed by Heine.[37]
To perform NMR calculations, good geometry is re-quired.[38] Starting from model building or downloaded coordinates,[39] we first optimized the geometries using the PM3 semiempirical method.[40] Frequency analyses were normally performed at this level to ensure that the structures are indeed minima. The structures corresponding to true minima were further optimized using B3LYP functional[36] and the STO-3G, 3-21G, and 6-31G*(or 6-31G for C86, C88, and C90) basis sets. The main purpose of this practice is to save computer time by always starting from the best available geometry; it also gives structures and energy values at the intermediate theoretical levels. Geometry optimizations normally converge after about ten cycles at the B3LYP/STO-3G level and after about four or five cycles with larger basis sets. Because the numbers of IPR isomers for the large full-erenes are very large (46 for C90),[13] we have limited the calculations using the larger basis sets on the isomers of fullerenes C86, C88 and C90 that have relative energies less than 25 kcal/mol at the B3LYP/STO-3G level. The default settings for convergence criteria and the default integration grid were used. All geometry optimizations were performed using Gaussian 98 program.[41]
The NMR shielding tensors were evaluated employing the GIAO method[42- at the geometries optimized at the highest level. The 6-31G* or 6-31G basis sets were used for all fullerene isomers depending on the basis set used for geometry optimization. A larger 6-311G** basis set was also used for selected isomers. The NMR calculations were carried out using the PQS program.
The 13C NMR chemical shifts were obtained by converting the isotropic shielding constant by the following formula:
where the experimental chemical shift of C60, d(C60), is taken as 143.15 ppm, after Avent et al.,[44] s(C60) is the calculated chemical shielding for C60, and i is the carbon atom under consideration. The experimental chemical shift of C60 is used as a second reference to correct the systematic error of about 4 ppm in the calculated chemical shifts when only TMS is used as the reference.[14]
VALIDATION OF PREDICTED 13C NMR CHEMICAL SHIFTS FOR FULLERENES
In this section, we show that the DFT-calculated 13C NMR chemical shifts for fullerenes agree well with experimental values for the fullerene isomers that can be identified based on the 1-D NMR pattern. It is shown that the spectral span, grouping of the peaks, and the relative positions of the half-intensity peaks with respect to those of full-intensity peaks are important features in comparing theoretical and experimental NMR spectra. An empirical trend emerges to indicate that the NMR peaks above 140 ppm are better reproduced than peaks below 140 ppm.
Buckminsterfullerene C60
The icosahedral symmetry of fullerene C60 renders all of its 60 carbon atoms equivalent, giving one signal in the NMR spectrum. The observed chemical shift for C60 ranges from 142.68[4] to 143.2 ppm.[5,8,10] The chemical shift predicted at the B3LYP/6-31G* level is 138.72 ppm.[14] This is in very good agreement with experiment, considering that 13C NMR signal spread over a wide range of 0-200 ppm. The deviation between our prediction and experiment is 3.96 to 4.48 ppm depending on which experimental value is used. This difference is comparable to deviations of earlier theoretical values,[34-ranging from ~ 6 ppm for GIAO-HF to ~ 1 ppm for GIAO-UDFT-BPW91 methods. Because this deviation is mainly systematic for fullerenes,[31] we use the chemical shift of C60 as a second reference in calculating chemical shifts for other fullerenes.
Fullerene C70
Fullerene C70 has D5h symmetry that gives five different sets of atoms. Five NMR peaks were observed in experiment and assigned to the five sets of atoms based on both 1-D and 2-D NMR measurements.’4,5,7- The NMR spectrum of C70 calculated at the B3LYP/6-31G* level of theory correctly shows the required two full-intensity peaks and three half-intensity peaks.[14] Fig. 2 shows the experimental and predicted NMR spectra for C70. The calculated spectral span and the distribution of the peaks are in good agreement with experiment. Similar results were also reported by Buhl et al.[34] and Heine et al.[31,32] Because all five peaks have been experimentally assigned, the case of C70 presents a unique example to assess the quality of the predicted chemical shifts. A one-to-one comparison gives 0.70, 0.24, — 0.26, 1.09, and 1.32 ppm deviations with respect to the experimental values of Taylor et al.[4] Because the chemical shifts are referenced with respect to the experimental value of C60 at 143.15 ppm and this is the center of the normal spectral span of fullerenes, it is expected that peaks far away from the spectral center have larger deviations than those close to the center. It seems, however, that the peaks below 140 ppm have larger deviations from experimental values than those peaks above 140 ppm. In the case of C70, the deviation for the most upfield peak (1.32 ppm) is almost twice that for the most downfield peak (0.70 ppm). This trend becomes clear when we consider more NMR spectra of fullerenes. Therefore we expect a 1-2 ppm overesti-mation for peaks below 140 ppm and a much better comparison for peaks above 140 ppm.
Fig. 2 13C NMR spectra of C70 by (i) experiment,’4- (ii) experiment,’10 and (iii) calculated by B3LYP/6-31G*. All spectra are referenced to C60 at 143.15 ppm.
Fullerene C76
Two IPR structures are possible for fullerene C76,[13] but only one isomer with D2 symmetry has closed-shell electronic configuration, thus stable under normal conditions. Experimentally, 19 NMR peaks have been reported with equal intensity.[8] The 19 peaks form five groups from downfield to upfield, containing 1, 6, 8, 3, and 1 peaks. These five groups and the spectral span were well reproduced in the predicted NMR spectrum,[14] as shown in Fig. 3. Fifteen peaks occurring between 140 and 150 ppm appear to agree with experiment much better than the four peaks below 140 ppm. Overestimations of chemical shifts of as much as 2.22 ppm from experiment were calculated for the four peaks below 140 ppm. The chemical shifts of C76 earlier predicted by IGLO-DFTB were able to give a good estimate on the spectral span but failed to show the subtle distribution of the peaks.[31,32] A one-to-one assignment of the NMR peaks based on our predicted chemical shifts was not possible because of the crowdedness of the spectrum. It is, however, obvious that the NMR chemical shifts calculated at the B3LYP/6-31G* level are accurate enough to determine the isomeric structure when good experimental spectrum from isomer-pure sample is available.
Fig. 3 13C NMR spectra of C76:1 by (i) experiment,[8] (ii) experiment,’10-1 (iii) experiment,’12-1 and (iv) calculated by B3LYP/6-31G*. All spectra are referenced to C60 at 143.15 ppm.
Fullerene C78
Three of the five IPR isomers of C78 have been characterized by NMR spectroscopy.1-9-12-1 Isomer 1 (D3) has 13 NMR peaks with equal intensity, which can be divided into four groups with 2, 2, 7, and 2 peaks from downfield to upfield. The predicted NMR chemical shifts gave good agreement with experiment in terms of distribution of peaks and spectral span.[14] It became obvious from our theoretical result that two NMR peaks occur in the 132135 ppm region instead of three as reported in Ref. [10]. The deviations from experiment for the two most down-field peaks, 0.18 and — 0.17 ppm, are much smaller than those for the two most upfield peaks, 1.70 and 0.89 ppm, illustrating the much better reproduced chemical shifts for peaks above 140 ppm.
Isomers 2 and 3 both have C2v symmetry, but show different NMR patterns: the ratio of full-intensity peaks: half-intensity peaks is 18:3 for isomer 2 and 17:5 for isomer 3. The spectral spans were well reproduced for these two isomers by our DFT calculations.1-14-1 The distribution of the peaks was also in agreement with experiment, thus confirming earlier assignment of the isomeric structures. Also reported were the chemical shifts for isomers 4 and 5 of C78, which could facilitate their identification if they are observed.
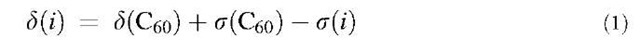

![13C NMR spectra of C76:1 by (i) experiment,[8] (ii) experiment,'10-1 (iii) experiment,'12-1 and (iv) calculated by B3LYP/6-31G*. All spectra are referenced to C60 at 143.15 ppm. 13C NMR spectra of C76:1 by (i) experiment,[8] (ii) experiment,'10-1 (iii) experiment,'12-1 and (iv) calculated by B3LYP/6-31G*. All spectra are referenced to C60 at 143.15 ppm.](http://what-when-how.com/wp-content/uploads/2011/03/tmp1CF27_thumb_thumb.jpg)
