Fullerene C80
The NMR spectra of six IPR isomers of C80 predicted by DFT calculations have been reported, including isomers 1-5 and a D2 distorted form of isomer 7(Ih).[45] The NMR spectra of isomer 2 (D2) show good agreement between theory and experiment.[30] At the downfield end, a group of three peaks were well reproduced in the spectra calculated using B3LYP hybrid functional and the 6-31G, 6-31G*, and 6-311G** basis sets. Sixteen peaks occurring at the middle of the spectrum in the calculated spectra also agree with experiment. One peak at the upfield end tends to be underestimated by large basis set than by medium basis set. Overall, the distribution for most of the peaks was well reproduced.
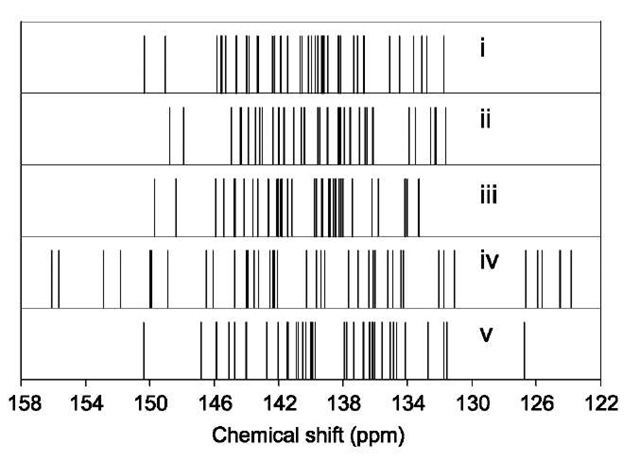
Fig. 4 13C NMR spectra of C80:1 by (I) experiment,[46] (II) calculated by B3LYP/6-31G*, (III) by B3LYP/6-311G**, (IV) HF/6-31G*, and (V) BP86/SVP.[48] All spectra are referenced to C60 at 143.15 ppm.
The NMR spectrum of another observed isomer, C80:1, shows large discrepancy between DFT results[45] and experiment,[46] as shown in Fig. 4. This isomer has D5d symmetry, thus shows three full-intensity peaks and two half-intensity peaks. The DFT-predicted spectrum shows that the two half-intensity peaks occur at down-field positions than the full-intensity peaks, which is consistent with experiment. The separation within the four downfield peaks seems to be comparable with that in experiment. However, one full-intensity peak in the DFT results occurs at around 90 ppm, far below the normal NMR ranges for fullerenes. Switching to a gradient-corrected pure functional such as BLYP did not improve the result.[47] Furche and Ahlrichs[48] also reported similar chemical shifts for the D5d isomer of C80 using the BP86 functional.
To solve the apparent discrepancy, we have carried out NMR calculations at the HF/6-31G* level.[47] The chemical shifts are all in the 120-155 ppm range that is normal for fullerenes, and the peak positions are in good agreement with experiment. On the energetic aspect, isomer 1 is predicted at the B3LYP/6-31G* level to be 2.20 kcal/mol less stable than isomer 2, but its HOMO-LUMO gap is only 0.986 eV, which is much smaller than that of isomer 2 (1.346 eV). At the HF level, its HOMO-LUMO gap is 4.56 eV, still slightly smaller than that (4.97 eV) of isomer 2, but much larger than those 3.2 eV) of isomers 3-5. This shows that the wrong chemical shifts from DFT calculations can be related to the severely underestimated HOMO-LUMO gap.
Isomers 3, 4, and 5 have small HOMO-LUMO gaps and high relative energies at both DFT and HF levels.[45,47] These isomers also have NMR peaks at beyond the normal 120-160 ppm range, suggesting that the electron distribution in the molecule is less optimal.
Fullerene C84: Two Major Isomers
Two major isomers of C84 were among the first observed fullerene species[11,12,49] and have drawn much research interest. Because isomer 23 (D2d) can be uniquely identified by 1-D NMR spectrum among the 24 possible IPR isomers[13] and isomer 22 (D2) has been identified by 2-D NMR experiment on the 13C-enriched sample,[50] they represent interesting testing cases for the DFT-calculated chemical shifts. We used density functional theory at the B3LYP level with the 6-31G* and 6-311G** basis sets to predict the chemical shifts for these two isomers and isomer 21 (D2).[15] Isomers 22 and 23 were isoenergetic and both have large HOMO-LUMO gaps. Fig. 5 shows their theoretical NMR spectra in comparison with experiment. Both DFT results for isomer 23 are in very good agreement with experiment in terms of the spectral span and peak positions. The rms deviation calculated for all 11 chemical shifts were 0.464 and 0.556 ppm for 6-31G* and 6-311G** basis sets, respectively. We note that because one-to-one peak assignment was not possible, the rms deviation will likely be larger when the peaks are assigned. However, the change would be small and thus it would not affect our conclusion. It is evident that the accuracy of the calculated chemical shifts allows identification of isomeric structures of fullerenes.
Fig. 5 Theoretical and experimental 13C NMR spectra of C84. (a) Spectra of 23(D2d) by experiment (i), B3LYP/6-31G* (ii), and B3LYP/6-311G** (iii). (b) Spectra of 22(D2) by experiment (i), B3LYP/6-31G* (ii), and B3LYP/6-311G** (iii), and of 21(D2) by B3LYP/6-31G* (iv), and B3LYP/6-311G** (v). Experimental spectra reproduced from Ref. [52].
Earlier calculated NMR results could not unambiguously distinguish between isomers 21 and 22.[29]31,32] Our DFT-calculated chemical shifts, as shown in Fig. 5b, clearly differentiate these two isomers.[15] Both spectral span and peak distribution conclusively favor isomer 22 over isomer 21 as the observed species. The rms deviations of chemical shifts between theory and experiment were 1.944 and 0.512 ppm for isomers 21 and 22 at the B3LYP/6-31G* level, respectively. Essentially same values of rms deviation were obtained from calculations using a larger basis set 6-311G**. Again, we note that, while the rms deviation values will likely change when all NMR peaks are assigned, the conclusion will hold. This assignment was further supported by the large differences in the relative energies (16.04 kcal/mol) and the HOMO-LUMO gaps of isomers 21 and 22.
IDENTIFICATION OF FULLERENE ISOMERS BY NMR SPECTRA
As the results for the nine observed isomers of fullerenes C70 to C84 have shown, the DFT-calculated NMR chemical shifts are accurate enough to determine the molecular structures for the observed fullerene isomers. In the mean time, care needs to be taken in the cases where the DFT-calculated HOMO-LUMO gap is too small. In the following section, we summarize theoretical NMR results for fullerenes C82 to C90 and for charged and substituted ful-lerene species, focusing on the identification of the observed isomers when experimental spectra of good quality are available. The calculated NMR chemical shifts for the isomers that have not been isolated have also been reported in the literature and should facilitate in determining the molecular structures when new isomers are observed.
Fig. 6 13C NMR spectra of C2 isomers of fullerene C82 for (i) isomer 3 by experiment,111- (ii) isomer 3 calculated by B3LYP/6-31G, (iii) isomer 3 calculated by B3LYP/6-31G*, (iv) isomer 1 calculated by B3LYP/6-31G*, and (v) isomer 5 calculated by B3LYP/6-31G*. All spectra are referenced to C60 at 143.15 ppm.
Fullerene C82
Fullerene C82 poses the first challenge to successively identify an isomer based on theoretical 13C NMR chemical shifts. One major isomer having C2 symmetry was observed in experiment with some minor isomers whose symmetry could not be determined.1-11-1 Of the three C2 isomers (1, 3, and 5), isomer 3 was predicted to have the lowest energy and a large HOMO-LUMO gap from our DFT calculations.1-51-1 Its predicted chemical shifts compare favorably with experiment, as shown in Fig. 6. The two NMR peaks at the downfield end of the spectrum are well separated from the rest in both theory and experiment. The separation of a group of six peaks at the upfield end from the group at the middle of the spectrum is less obvious in the 6-31G* results than in the 6-31G results. The spectral spans from theoretical results of both basis sets also show isomer 3 as the observed isomer. Thus the excellent agreement of the NMR spectrum together with the low energy and large gap allowed us to identify the observed C2 isomer as isomer 3.
The predicted chemical shifts of isomers 1, 2, 4, and 5 fell in the normal range for fullerenes, whereas the chemical shifts of isomer 6 have values as low as 113.50 ppm.[51- Because the four isomers have relatively low energy and large HOMO-LUMO gaps, their observation may be possible and the predicted chemical shifts could facilitate their identification.
Fullerene C84
Nine isomers have been obtained and characterized by NMR spectroscopy.1-11,12,49,52-54-1 As discussed above, structures of the two major isomers 22 (D2) and 23 (D2d) of fullerene C84 have been assigned,[15] and here we look at the minor isomers.
A second D2d isomer of C84 was observed and assigned as isomer 4 based on the ratio of the full-intensity peaks: half-intensity peaks.[53] In our DFT results at the B3LYP/ 6-31G* level, isomer 4 has a relative energy of 14.64 kcal/ mol and a HOMO-LUMO gap of 2.13 eV.[55] The predicted NMR chemical shifts were in good agreement with experiment. Three full-intensity peaks are well separated from each other at the 131-138 ppm region, while the rest of the full-intensity peaks are found at 143149 ppm. The half-intensity peaks are all above 140 ppm, consistent with experiment.
A second D2 isomer was tentatively assigned as isomer 5.[53] Its experimental NMR spectrum has a single peak well separated from others at the downfield end and four peaks separated at the upfield end. Our theoretical spectrum of isomer 5 reproduced these features very well, confirming the earlier assignment.[55] The calculated spectral span, 21.17 ppm, is in excellent agreement with the measured value (22.10 ppm). Similarly, good agreement was also obtained for the spectral span (15.13 ppm) of the observed C2 isomer and that of isomer 11 (15.15 ppm), whereas the calculated spectral spans for other C2 isomer are much larger.
Two Cs isomers were isolated from graphite soot of DC arc discharge.[53] Both isomers have two half-intensity peaks and 41 full-intensity peaks, which suggests that they are isomers 14 and 16 but no final assignment could be reached. The spectral spans are very close, being 15.29 and 15.51 ppm for Cs(a) and Cs(b), respectively, and the full-intensity peaks are very crowded. Because of this, the assignment of these isomers has to be based on the chemical shifts of the half-intensity peaks. The two half-intensity peaks of isomer 14 occur at 132.22 and 148.49 ppm in our B3LYP/6-31G* results,[55] in good agreement with those of Cs(b) occurring at 134.33 and 148.44 ppm. On the other hand, the two half-intensity peaks of isomer 16 occur at 136.12 and 141.01 ppm, consistent with those of Cs(a) appearing at 137.03 and 141.76 ppm. Thus isomer Cs(a) is isomer 16 and isomer Cs(b) has the structure of isomer 14.
Two highly symmetric isomers, 19 (D3d) and 24 (D6h), have also been characterized.1-54-1 The predicted general pattern of the six full-intensity peaks of isomer 19 was in agreement with the experiment.1-55-1 The two half-intensity peaks appear at 133.85 and 136.86 ppm, which is different from experimental values of 136.39 and 147.81 ppm. Other authors have suggested that the half-intensity peak at downfield region is misassigned.[32] This appears to be likely because the measurement was taken from mixture samples. For isomer 24, the two full-intensity peaks are in fair agreement between theory and experiment,[55] whereas the position of one of the three half-intensity peaks is shown to be misassigned in experiment. Future NMR measurement on isomer-pure samples is highly desirable for these isomers.
Fullerene C86
Two isomers of C86 with C2 and Cs point group symmetry have been isolated using the multistage HPLC method.[56] Based on earlier energy calculations,[57] they were provisionally assigned as isomers 17(C2) and 16(Cs), respectively. Our calculated NMR chemical shifts at the B3LYP/ 6-31G level confirmed this assignment.[58] The calculated spectral span, 20.25 ppm, of isomer 17 is in excellent agreement with the experimental value, 20.28 ppm. The other C2 isomers (2, 3, 4, 6, and 14) all have much larger spectral spans. A single isolated peak at the downfield end, a single isolated peak at the upfield end, and the crowded middle part of the NMR spectrum are well reproduced in our theoretical results for isomer 17.
Two Cs IPR isomers, 15 and 16, have the ratio of full-intensity peaks/half-intensity peaks[13] that was observed in experiment. In addition to the low energy and large HOMO-LUMO gap, isomer 16 has a calculated spectral span of 17.73 ppm, in good agreement with experimental value of 17.99 ppm for the Cs isomer. The distribution of the NMR peaks also supports the experimental assignment.[58]
Fullerene C88
Three isomers of C88 were separated and characterized by NMR as C88-1(Cs), C88-2(C2), and C88-3(C2).[56] Isomer 17(Cs) has the lowest energy and a large HOMO-LUMO gap in our DFT results.[59] The chemical shifts of isomer 17 calculated by B3LYP/6-31G have two distinct half-intensity peaks at the downfield end, which is consistent with experimental spectrum. The 42 full-intensity peaks occur within the 130-147 ppm range in theory, comparing well with the experimental range of 131-148 ppm. Similar comparison was also obtained for the 6-31G* results.
Of the five C2 isomers studied, isomers 7 and 33 have low energies and have been proposed as the observed isomers.[56] All C2 isomers have 44 NMR peaks with equal intensity. The spectral spans for isomers 7 and 33 are 20.68 and 18.29 ppm,[59] respectively, suggesting that they correspond to C88-2 and C88-3, respectively. Detailed comparison on the peak positions shows that two peaks at the downfield end of the B3LYP/6-31G*-calculated spectrum of isomer 7 are separated from others, which agrees with the spectrum of C88-2. The downfield end of the predicted NMR spectrum of isomer 33 is crowded, in consistency with the spectrum of C88-3. Thus our DFT results strongly suggest C88-2 as isomer 7 and C88-3 as isomer 33.
Fullerene C90
Isomer 45(C2) of C90 is the most stable isomer in our DFT results, while isomers 28(C2), 30(C:), 32(C:), 35(Cs), 40(C2), and 46(C2v) show significant stabilities.[60] The chemical shifts for these isomers all occur in the normal range of 125-155 ppm for fullerenes. Isomers of C90 have been observed only in mixture, and this prevents the determination of molecular structures because of the large number of NMR peaks. Five weak experimental NMR peaks were reported in literature, which led to the speculation of the existence of isomer 36(C2v).[61] Our DFT calculations show that isomer 36 has very high energy, thus unlikely to be the source of the observed weak NMR peaks. A comparison of the predicted chemical shifts suggests that the five weak peaks are more likely from the stable isomers 35 and 46.[60] Future measurements on isomer-pure samples are needed to elucidate the ground-state structures of fullerene C90.
Charged and Substituted Fullerenes
Besides the pristine fullerenes, the charged and substituted fullerenes have also been subjected to theoretical NMR studies using DFT methods, and the NMR chemical shifts calculated at B3LYP/6-31G* or comparable levels of theory have been used for identification of the fullerene species. The hexa-anions of fullerenes C76-D2, C78-D3, C78-C2v, C84-D2, and C84-D2d have been recently obtained from lithium reduction of neutral fullerenes and characterized by NMR spectroscopy.1-62-1 DFT-calculated chemical shifts were used to assist the determination of the reduction states of the multiply charged anions. The chemical shifts of the hexa-anions are deshielded comparing to the neutral fullerenes.
The carbocation C59N+ has recently been obtained from the oxidation of (C59N)2 by crude hexabromo(N-phenyl)carbazole and characterized by MS, X-ray, NMR, UV-Vis, and infrared spectroscopy.1-63-1 Theoretical NMR chemical shifts calculated at the B3LYP/6-311G** level are in good agreement with experiment. The majority of the NMR peaks cluster around 144 ppm, showing the overall positive charge of the cation.
The 13C NMR chemical shifts of the proposed aza-[60]fullerene C48N12[64] have been recently predicted using DFT method.[65] Compared with C60, most NMR peaks of C48N12 have lower chemical shifts.
NMR CHEMICAL SHIFTS AND LOCAL GEOMETRY
Nuclear magnetic resonance chemical shifts are determined by the electronic configuration of the molecule and are affected by the local structure close to the atom. Diederich et al.[8]10] have suggested that the carbon site in fullerenes be divided into three types: pyracylene (type 1, pc), corannulene (type 2, cor), and pyrene (type 3, py).
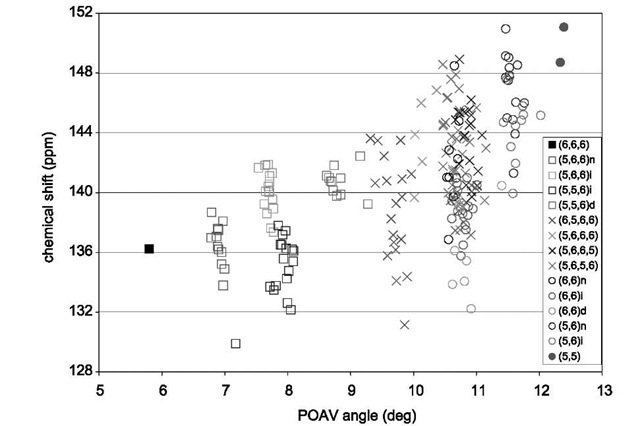
Fig. 7 Theoretical 13C NMR chemical shifts and POAV angles of isomers 4, 5, 11,14, 16,19, and 22-24 of fullerene C84 calculated by B3LYP/6-31G*. The py-type carbons are represented by squares, the cor type are represented by crosses, and the pc type are represented by circles.
The chemical shifts should fall in the pc>cor>py order. Heine et al.[31,32- plotted the chemical shifts against the p-orbital axis vector (POAV) angle1-66-1 and found a marked upward trend of chemical shifts with increasing POAV angle for pc and cor sites but no apparent trend for py sites.
We have also studied the relationship between chemical shift and local geometry in the context of POAV angle.1-14,45,51,55-1 The chemical shifts are, in general, larger for type pc than cor, which in turn are larger than py. There is, however, no clear range where each type will occur and the chemical shifts of different types are in fact often mixed. To thoroughly study the relationship between chemical shift and local geometry, we have considered further neighbors of a given atom and arrived at 15 structural motifs.1-55-1 Fig. 7 shows the graph of chemical shifts vs. POAV angles for the nine observed isomers of C84. Distinct ranges of POAV angles and chemical shifts are seen for each of the five motifs of the py type. Two and three groups of POAV angles are obvious for the cor and pc types, respectively, but the chemical shifts for these sites occur throughout the 130-150 ppm range.
ENERGETICS OF STABLE FULLERENES
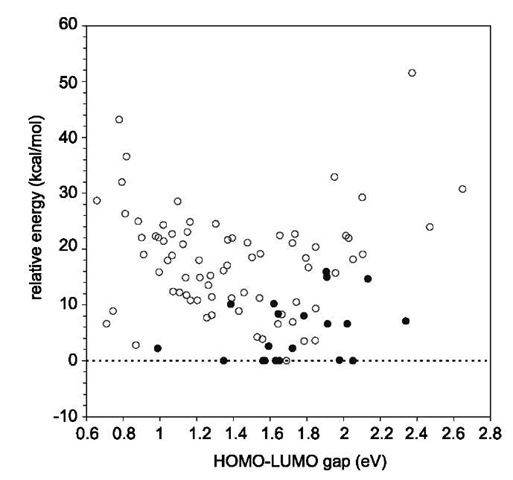
We have been using low relative energy and large HO-MO-LUMO gap as indicators of the stability of fullerene isomers. Here we show a statistical graph of the energy and gaps for the observed isomers in Fig. 8. The majority of the observed isomers have relative energies less than 10 kcal/mol at the B3LYP/6-31G* level. The highest energy of the observed isomers is for C84:5 with 15.94 kcal/mol. The HOMO-LUMO gaps for observed isomers are all in the range 1.35-2.34 eV. Note that the gap (0.99 eV) for C80:1 is wrong in the DFT calculations. Therefore for a fullerene isomer to be isolable, it should have a low relative energy and large HOMO-LUMO gap.
Fig. 8 The relative energy and HOMO-LUMO gap for the isomers of fullerenes C78 to C90. The observed isomers are represented by solid circles.
CONCLUSION
Theoretical NMR chemical shifts calculated using density functional theory at the B3LYP/6-31G* level have been shown to be accurate to unambiguously determine the molecular structures of the observed fullerene isomers. For the isomers of fullerenes C70, C76, C78, C80, and some isomers of C84 where the NMR spectral patterns uniquely correspond to the structures, the predicted chemical shifts are in very good agreement with experiment. The spectral spans and the distribution of the NMR peaks are well reproduced for these cases. Comparing the predicted and experimental chemical shifts of isomers 22 and 23 of fullerene C84, rms deviations were 0.464 and 0.512 ppm, respectively. The calculated NMR chemical shifts allowed the identification of the previously unassigned fullerene isomers: C82:3, C84:5, C84:11, C84:14, C84:16, C84:22, C86:16, C86:17, C88:7, C88:17, and C88:33. The calculated NMR chemical shifts also proved necessary in determining the charge status of the charged and substituted ful-lerene species. The local geometry at the sp2-hybridized carbon in fullerenes has been shown to have some effect on the chemical shift, but lack any linear relationship. The observed fullerene isomers all have low energies and large HOMO-LUMO gaps among the set of possible IPR iso-mers for each fullerene family. Because of the high accuracy of the DFT-calculated chemical shifts, we expect the calculation of NMR chemical shifts to be performed commonly in various areas of chemical research because the computers are becoming faster and the programs capable of calculating chemical shifts are becoming easier to use.
![13C NMR spectra of C80:1 by (I) experiment,[46] (II) calculated by B3LYP/6-31G*, (III) by B3LYP/6-311G**, (IV) HF/6-31G*, and (V) BP86/SVP.[48] All spectra are referenced to C60 at 143.15 ppm. 13C NMR spectra of C80:1 by (I) experiment,[46] (II) calculated by B3LYP/6-31G*, (III) by B3LYP/6-311G**, (IV) HF/6-31G*, and (V) BP86/SVP.[48] All spectra are referenced to C60 at 143.15 ppm.](http://what-when-how.com/wp-content/uploads/2011/03/tmp1CF28_thumb_thumb.jpg)
![Theoretical and experimental 13C NMR spectra of C84. (a) Spectra of 23(D2d) by experiment (i), B3LYP/6-31G* (ii), and B3LYP/6-311G** (iii). (b) Spectra of 22(D2) by experiment (i), B3LYP/6-31G* (ii), and B3LYP/6-311G** (iii), and of 21(D2) by B3LYP/6-31G* (iv), and B3LYP/6-311G** (v). Experimental spectra reproduced from Ref. [52]. Theoretical and experimental 13C NMR spectra of C84. (a) Spectra of 23(D2d) by experiment (i), B3LYP/6-31G* (ii), and B3LYP/6-311G** (iii). (b) Spectra of 22(D2) by experiment (i), B3LYP/6-31G* (ii), and B3LYP/6-311G** (iii), and of 21(D2) by B3LYP/6-31G* (iv), and B3LYP/6-311G** (v). Experimental spectra reproduced from Ref. [52].](http://what-when-how.com/wp-content/uploads/2011/03/tmp1CF29_thumb1_thumb.jpg)