INTRODUCTION
The discovery of carbon nanotubes (CNTs) in the early 1990s has stimulated intensive research to characterize their structure and properties. The outstanding electronic and mechanical properties suggest that CNTs may find application in electronic devices, composite materials, energy storage, field emission, biology, medicine, and chemical sensors. Most of these applications take advantage of the structure and physical properties of CNTs, such as high mechanical strength, excellent thermal and electric conductivity, high aspect ratio, and the hollow cavity. Chemical functionalization has been employed to tailor the properties of CNTs and to enhance the ability to manipulate CNTs.
CNTs contain only conjugated carbon atoms and therefore they are chemically inert, and this is reflected by their properties such as insolubility in all solvents and low chemical reactivity. The inert nature of CNTs limits the available identification and characterization tools, and hinders the manipulation and application of carbon nanotubes. Chemical functionalization of CNTs is a useful tool for addressing these problems, and functionalized CNTs have found broad application, including composite materials, sensors, and biomaterials. In the present article we review the structure and properties of CNTs, but in the main we focus on the chemistry of carbon nanotubes including previous work and recent development.
STRUCTURE AND PROPERTIES OF CARBON NANOTUBES
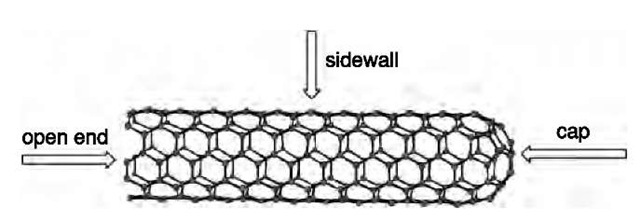
CNTs can be classified as single-walled carbon nano-tubes (SWNTs) and multiwalled carbon nanotubes (MWNTs). The structure of an individual SWNT can be regarded as a two-dimensional (2-D) graphene sheet cut at various angles with respect to the hexagonal lattice that has been wrapped up into a seamless cylinder, which is capped by fullerene hemispheres at both ends (Fig. 1).
MWNTs are composed of concentric cylinders placed around a common central hollow, with spacing between the layers close to that of the interlayer distance in graphite 0.34 nm).
CNTs can be metallic or semiconducting, depending on their diameter and chirality. ”Armchair” tubes have energy bands that cross the Fermi level and are therefore metallic. ”Chiral” and ”zigzag” nanotubes are expected to be metallic when n — m=3l (where l is an integer) or semiconducting with an energy gap of order ~ 1 eV when n — m^ 3l. n and m are integers of the vector equation R=na 1+ ma2, where a 1 and a2 are the unit cell vectors of a graphen sheet. Fig. 2 shows the electronic density of states (DOS) of a metallic and a semiconducting SWNT close to Fermi level. For the metallic SWNT, there is a finite value of the DOS at the Fermi level. On the other hand, a complete gap between the spikes close to Fermi level can be found in the semiconducting SWNT. The energy separation between each pair of peaks represents the band gap of SWNTs. By using simple tight-binding (STB) theory, in which the electronic band structure is assumed to arise from a pure p-orbital at each conjugated carbon atom, the low-energy band gap transitions take a simple analytical form: S11=2ab/d, S22=4ab/d, M11 = 6ab/d, where a is the carbon-carbon bond length (0.142 nm), b is the transfer or resonance integral between the p- and p-orbitals (b=2.9 eV), and d is the diameter (nm) of the particular semiconducting or metallic SWNT. S11 and S22 are the first and second pairs of singularities in the DOS of semiconducting SWNTs, respectively. M11 corresponds to the transition between the first pair of singularities in the DOS of metallic SWNTs.[1-3] The characteristic interband electronic transitions provide the spectroscopic signature of the SWNTs in the near-IR (NIR)/VIS spectral region (Fig. 2). As most SWNT preparations are a mixture of metallic and semiconducting SWNTs, the interband transitions arising from both species are visible.
Besides these unique electronic properties, CNTs exhibit excellent mechanical and thermal properties.
Fig. 1 Structure of the single-walled carbon nanotube.
CNTs exhibit the highest Young’s modulus and tensile strength of all known materials. The Young’s modulus for an individual (10, 10) SWNT is – 0.64 TPa[4] and up to 1.47 TPa for a 15-SWNT rope,[5] which is much higher than that of conventional carbon fibers which have values in the range of 200-800 GPa.[6] Young’s modulus of MWNTs was reported to be 1-2 TPa,[7] and the bending strength of individual MWNTs reaches 28.5 GPa.[8] The measured thermal conductivity of an individual MWNT (— 3000 W/m*K) is larger than that of natural diamond and the basal plane of graphite (both 2000 W/m*K).
CHEMISTRY OF CARBON NANOTUBES
The covalent chemistry of CNTs may be classified into two categories.1-9-1 The first involves functionalization at the ends or at defect sites of the CNTs, where there exists the possibility to add to the carbon nanotube at a a-bonded site, without attacking the p-system; this type of chemistry usually involves reaction at a previously introduced carboxylic acid group. These carboxylic acid groups are usually generated by nitric acid treatment and can be used as precursors in functionalizations such as amidation and esterification.[10,11- The second type of covalent chemistry involves sidewall functionalization in which the carbon-carbon double bonds on the sidewall of CNTs react. Such reactions include fluorination,[12] carbene1-9’11’13’14-1 or nitrene addition, Birch reduction,[9'15] 1,3-dipolar addition,1-16-1 and reactions with radicals.[17-19]
Purity Evaluation of SWNTs
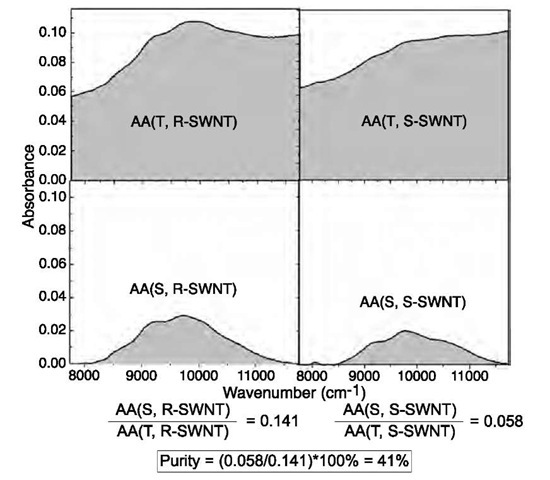
In current preparations, a large amount of impurities, such as metal catalysts, amorphous carbon, and carbon nano-particles, are generated during SWNT production. In order to develop a rigorous macromolecular science of CNTs it is necessary to work with materials of the same high level of purity that is found in the pharmaceutical, electronics, and polymer industries. An obvious first step is the development of quality control and quality assurance criteria for the bulk purity of CNTs. Recently, a method was reported to quantitatively evaluate the carbonaceous purity of electric arc produced as-prepared SWNT soot by using solution-phase near-IR spectroscopy (NIR), in which the purity of a SWNT sample (X-SWNT) was evaluated against a reference sample (R-SWNT) by using the region of the second interband transition (S22) for semiconducting SWNTs;[20-22] whereas the metal content is obtained by thermogravimetric analysis.[22- As illustrated in Fig. 3, AA(T, R-SWNT) and AA(T, S-SWNT) (areal absorbance) are obtained by integration of the NIR spectra between the spectral cutoffs of SCL=7750 and SCH=11,750 cm"1, whereas AA(S, R-SWNT) and AA(S, S-SWNT) represent the area under the S22 absorption after baseline correction. In the example shown in Fig. 3, the carbonaceous relative purity of S-SWNT is then obtained as:
Fig. 2 Interband transition of arc-produced SWNTs near Fermi level.
Fig. 3 Purity evaluation of SWNTs.
This method is also applicable to the evaluation and optimization of SWNT purification procedures, and makes it possible to investigate the effects of purification treatment on SWNTs in a quantitative manner.[21,22]
Reactivity of SWNTs
The strain in nonplanar-conjugated organic molecules arises from two principal sources: pyramidalization of the conjugated carbon atoms, and p-orbital misalignment between adjacent pairs of conjugated atoms.[23-25] It is well known that the chemistry of the fullerenes is characterized by addition reactions.[26-29] The relatively high reactivity of C60 results from the conversion of trigonal carbon atoms (sp2-hybridized) to tetrahedral carbon atoms (sp3-hybridized). This serves to release the tremendous strain present in the spherodial geometry, because the pyramidalization angle 0p, which is related to the curvature at a conjugated carbon atom, of C60 (0p= 11.64°) is actually closer to the ideal tetrahedral angle (0p= 19.47°) than to the planar geometry required for trigonal hybridization (0p=O).[25,3O] Fig. 4 shows the pyramidalization angle of sp2- and sp3-hybridized carbon atoms. As a result of the spheroidal geometry there is no p-orbital misalignment in C60.
From the standpoint of chemistry, carbon nanotubes can be divided into two regions: the end caps and the sidewall. The origins of the strain in the end caps and the sidewall of CNTs are somewhat different. The end cap of a CNT can be regarded as a hemispherical fullerene, and the carbon-carbon bonds in this region of the carbon nanotube therefore experience a similar degree of strain due to pyramidalization to that of the equivalent fullerene. However, as the curvature in the sidewall of a CNT is much less than that in a fullerene of equivalent diameter, the carbon-carbon bonds in the sidewall of a carbon nanotube are much less reactive than those in the end cap.[9,31] In the fullerenes, pyramidalization is essentially the only source of strain because the p-orbital alignment is almost perfect because of the quasi-spheroidal geometry.[30,31]
Fig. 4 Pyramidalization angle (9p) of sp2 (left)- and sp3 (right)-hybridized carbon.
Fig. 5 The p-orbital misalignment angles (f) along the C1-C4 in the (5, 5) SWNT and fullerene.
In the case of the armchair CNT shown in Fig. 5, there are two types of carbon-carbon bonds in the sidewall: one is parallel to the circumference plane (which is perpendicular to the axis of the CNT), whereas the other lies at an angle to the circumference plane. As may be seen in Fig. 5, the first bond is reminiscent of the situation in the fullerenes with perfect alignment of the lobes of the p-orbitals, whereas the second bonding geometry requires a twist of the p-bond.[9,31] In the latter case, the angle between the lobes of the p-orbitals of the adjacent carbon atoms [p-orbital misalignment angle (f)] will differ from zero and this is a source of strain in the carbon nanotubes that is almost totally absent in the fullerenes. This strain due to orbital misalignment leads to a differentiation between the bonds in the CNTs that may be reflected in their relative reactivity.
In the foregoing discussion we have adopted the p-orbital axis vector (POAV) analysis, which was successful in the analysis of the electronic structure and chemistry of the fullerenes,[25] and applied it to the CNTs. This analysis relies on the local geometry of the structures; however, the CNTs possess an extended electronic structure and this leads to the development of energy bands, which in the case of the metallic SWNTs produces a finite DOS at the Fermi level;[13,32] the semiconducting SWNTs, of course, have an energy gap at the Fermi level. Thus there should be a marked difference in the reactivity of metallic and semiconducting SWNTs in chemical reactions that are initiated by electron donation of electron transfer processes.[9,19]
Covalent Functionalization
Oxidation of carbon nanotubes
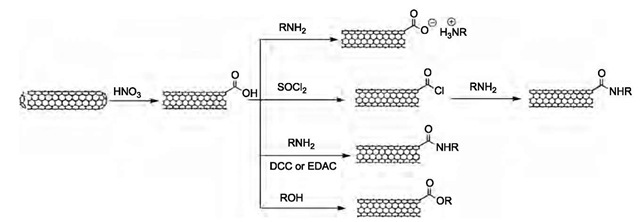
Oxidative processes were among the first chemical reactions to be applied to CNTs,[33] mainly for the purposes of purification and catalyst removal. Heating of CNTs in air at 700°C for 10 min leads to the opening of the hemispherical end caps of CNTs and demonstrated the different reactivities of the bonds in CNTs (end cap vs. sidewall). The controlled annealing of SWNTs in O2 is an effective method of purification.1-21-1 Other gas-phase oxidation methods including the use of H2S+O2[34] and O3[35] have also been reported. Liquid-phase oxidation processes, such as treatment with HNO3,[22,36-40] HNO3 + H2SO4,[10,40] H2SO4+KMnO4,[41] and KMnO4,[40] have been widely used in the processing of CNTs. These oxidants can remove the end caps of CNTs and introduce functional groups, such as -COOH, -OH, -C=O, at the dangling bonds.[40,42] Among these functionalities, the car-boxylic acid group is of great importance as it has been employed in a large number of CNT functionalization reactions. After nitric acid treatment of SWNTs, the concentration of carboxylic acid groups is about 1-3 at.% based on acid-base titration.[43]
End functionalization
As the carbon atoms at the ends have larger curvature than carbon atoms in the sidewall, they can be oxidized more easily than the other carbon atoms. The end (and defect) functionalization of the CNTs has important ramifications:
1. End functionalization does not destroy the extended p-network, thereby retaining the unaltered electronic and mechanical properties of the CNTs.
Fig. 6 Schematic representation of end functionalization of SWNTs.
2. End functionalization can dramatically change the chemical and physical properties of CNTs, enhance their solubility, and facilitate the manipulation of CNTs.
The carboxylic acid groups that terminate the ends and defect sites of SWNTs act as chemical precursors for the functionalization chemistry (Fig. 6). The first chemical synthesis of soluble SWNTs was reported in 1998.[11] The functionalization methodology was based on the amida-tion of SWNTs with a long-chain amine [octadecylamine (ODA)]. Acid-treated SWNTs were first reacted with thionyl chloride to form an acyl chloride intermediate.1-10-1 Then the acyl chloride intermediate was heated with ODA at 90°C to 100°C for 96 hr. The resulting amide form of SWNTs was soluble in many common organic solvents, including chloroform, dichloromethane, aromatic solvents (benzene, toluene, chlorobenzene), and CS2. The solubility of the s-SWNTs in 1,2-dichlorobenzene and CS2 is higher than 1 mg/mL. This important finding not only provides a new method for obtaining well-characterized highly purified SWNT materials by separating soluble SWNTs from insoluble impurities,[44,45- but also opened the door for the study of SWNT chemistry in the organic solution phase. Various solution spectroscopy can be applied to characterize the dissolved SWNTs.[11,46,47]
Based on the same method, other amines, such as 4-dodecyl-aniline,[46- glucosamine[48- and amine-rich polymers, dendra, and proteins,1-49-52-1 have been used to functionalize SWNTs. The methodology of these reactions is based on amidation via acyl chloride intermediate or the 1-ethyl-3(3-dimethylaminopropyl)cabodiimide (EDAC)- or 1,3-dicyclohexylcarbodiimine (DCC)-acti-vated reaction. The poly(propionylethylenimine-co-ethyl-enimine (PPEI-EI)-[51- and bovine serum albumin (BSA)-functionalized SWNTs[52- were quite soluble in water as well as organic solvents. Sonication during the reaction can promote EDAC-activated amidation.[51- The ring closure of SWNTs by reaction between the -COOH and -OH at their ends has also been reported.[53-
Solution-phase mid-IR spectroscopy had been used to estimate the ratio of the carbon atoms in the SWNT backbone to the carbon atoms in the end-groups and at defect sites of the ODA-functionalized soluble SWNTs.[47- By measuring the intensity of V(C-H) stretching vibrations that originate from the ODA alkyl chains and comparing with an external standard, the fractional amount of the alkyl chains in the soluble SWNTs samples can be determined. A quantitative C13 NMR study of ODA functionalized MWNTs has also been used to discuss the relationship between the content of ODA and the solubility of the CNTs.[54-