Synthesis and Properties of Carbon Fibers
SEM photographs together with schematic structural models are shown in Fig. 9 for typical carbon fibers: a high-strength polyacrylonitrile (PAN)-based fiber nearly parallel to the fiber axis, and this high degree of preferred orientation is responsible for their high modulus or stiffness as well as their relatively high graphitizability. The structures described above give rise to different physical properties, although each type of fiber features carbon hexagonal networks, possessing the strongest covalent bonds in nature (C-C bonds). These strong interatomic bonds lie in sheets essentially parallel to the fiber axis and are responsible for the high mechanical performance of these carbon fibers.
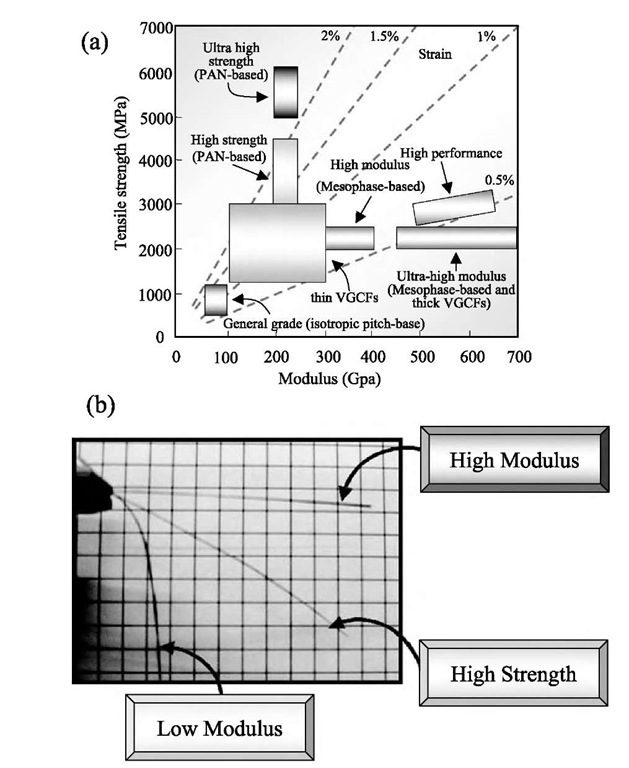
Fig. 10 (a) The mechanical properties of various kinds of carbon and graphite fibers[13'32] and (b) a direct comparison of the mechanical properties for high strength and high modulus fibers, where low modulus fiber droops under its own weight, but the high modulus fibers do not.
(Fig. 9a), a high-modulus PAN-based fiber (Fig. 9b), and a MPCF (Fig. 9c).[43,44] The PAN-based fibers consist of small sp2-carbon structural units preferentially aligned with the carbon hexagonal segments parallel to the fiber axis. This orientation is responsible for the high tensile strength of PAN-based carbon fibers.[45] By varying the processing conditions (e.g., oxidation conditions, choice of precursor material, and especially by increasing the heat treatment temperature) of PAN fibers, a better alignment of the graphene layers can be achieved (see the structural model of Fig. 9b), thus leading to stiffer, higher-modulus PAN fibers, but with lower strength.[44] PAN-based fibers are one of the typical hard carbons. MPCFs consist of well-aligned graphitic layers arranged Fig. 9 SEM micrographs of three types of carbon fibers and their corresponding structural models: (a) a high-strength PAN-based fiber, (b) a high-modulus PAN-based fiber, and (c) MPCF.[43'44] On the right-hand side of each fiber type, a schematic diagram of the fiber structure is shown.
Referring to Fig. 10a we see that PAN-based fibers have high strength and MPCFs have high modulus, while VGCFs provide mainly ultra-high modulus materi-als.[13,32] In this figure we also observe isotropic pitch-based (general grade) fibers, exhibiting much lower modulus and strength, but widely used in composites with cement matrix for construction because of their low cost and chemical stability. Fig. 10b demonstrates a direct indication of the differences in the mechanical properties of various carbon fibers, from low modulus-high strength to high modulus-low strength fibers from the lower left to the upper right in the photograph, where a yarn containing 500 fibers was initially placed in a horizontal position. These fibers are combined with other materials in order to design suitable mechanical properties and the fibers are used as a filler for various advanced composite materials. In order to get high performance in carbon and graphite fibers, it is very important to control the microstructure by selecting the appropriate organic precursor as well as the processing conditions.
Vapor-Grown Carbon Fibers
VGCFs have a very special structure such as annular-rings (see Fig. 11a) and are synthesized by a somewhat different formation process than that used to produce PAN-based and MPCFs. In particular, VGCFs are not prepared from a fibrous precursor, but rather from hydrocarbon gas, using a catalytic growth process outlined in Fig. 11b.[7-10,43,46] Ultra-fine transition metal particles, such as iron particles with a diameter of less than 10 nm, are dispersed on a ceramic substrate, and a hydrocarbon, such as benzene diluted with hydrogen gas, is introduced at temperatures of about 1100°C. Hydrocarbon decomposition takes place on the catalytic particle, leading to a continuous carbon uptake by the catalytic particle and a continuous output by the particle of well-organized tubular filaments of hexagonal sp2-carbon. The rapid growth rate of several tens of ,um/min, which is 106 times faster than that observed for the growth of common metal whiskers,[42] allows the production of commercially viable quantities of VGCFs. Evidence in support of this growth model is the presence of catalytic particles at the tips of the resulting VGCFs (Fig. 11c).[43] The primary thin hollow fiber is first formed by the catalytic process (with a diameter of less than several nanometers), and the fiber is then thickened by a successive chemical vapor deposition (CVD) process, corresponding to the deposition of pyrolytic carbon layers on the primary tubular core (Fig. 11d).
Fig. 11 (a) SEM image of vapor-grown carbon fibers (VGCFs),[43] (b) suggested growth mechanism of VGCFs using ultra-fine catalytic metal particles,[7] (c) very early stage of fiber growth in which the catalytic-particle is still active for promoting elongation growth. The primary fiber thus formed acts as a core for vapor-grown fibers.[43] (d) The fiber is obtained through a thickening process, such as the pyrolytic deposition of carbon layers on the primary thin fibers.[43] The encapsulated catalytic particle can be seen at the tip of the hollow core.
Fig. 12 (a) A vertical-type fiber synthesis system for the floating reactant method in which benzene vapor containing an organic-metallic compound such as ferrocene is introduced into a vertical-type reactor by hydrogen gas. The fibers grow while the catalytic particles are floating in the growth region of the reactor[13'43] and (b) VGCFs, obtained under controlled growth conditions, exhibit relatively homogenous diameter along the fiber length and also high purity.
Such a high growth rate of the primary core fiber provides the possibility of employing a 3-D growth process involving a floating catalyst in a reaction chamber without the presence of a solid substrate (see Fig. 12a). In this process, the nanometer-sized catalytic particles of metal can float (or be suspended) for a specified time in the hot zone of the reaction chamber in order to produce thin (100 nm homogeneous diameter) VGCFs of several hundred microns in length.[13,43] The resulting VGCFs sample shown in Fig. 12b consists of straight fibers with high purity, implying that the individual fibers experienced uniform reaction conditions as they passed through the reactor.[43] By proper choice of growth conditions, large quantities of high purity VGCFs can be obtained continuously. Furthermore, the fiber diameter can be varied through control of the residence time of the fiber in the reaction zone and of the pressure of the hydrocarbon feed stock. This basic process could be used for the large-scale production of carbon nanotubes by a CVD process based on the catalytic growth.[1a13,43,47-49]
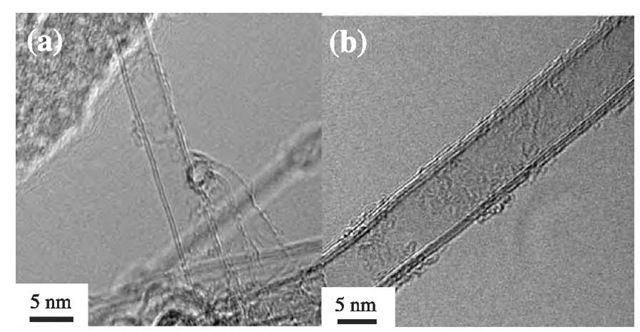
In Fig. 13a, we observe the broken edge of a thick VGCF. At the center of the annular ring structure, we can clearly observe an extruded carbon nanotube (with a diameter of ~ 5 nm), which serves as a template for growing the thicker carbon fiber.[11] This tubular core implies a discontinuity in the structure between the core and the thickened periphery of the carbon fiber. Such a discontinuity in structure is retained even after heat treatment at temperatures as high as 3000°C. This graphitization process introduces a fully developed graphite structure in the peripheral region of a VGCF exhibiting a polygonal shape (see Fig. 13b). Fig. 14a shows the early stage of fiber growth of a thin tubule corresponding to a SWNT, which has both a bare region and a region partially coated with pyrolytically deposited carbon.[50] Sometimes it is possible to observe an isolated carbon nanotube without a pyrolytic carbon coat at the early stage of formation (Fig. 14b). This type of nanotube can be produced by the same process as VGCFs by carefully controlling the vapor pressure of the hydrocarbon and using a much smaller size of catalytic particle.[50]
Fig. 13 (a) Carbon nanotube exposed at the breakage edge of a vapor-grown carbon fiber as grown (a) and heat-treated at 3000°C (b). The sample is fractured by pulverization and the core diameter is ~50 A. (b) These photos suggest a structural discontinuity between the nanotube core of the fiber and the CVD-deposited carbon layers. The images show the strong mechanical properties of the nanotube core, which maintains its form after breakage of the periphery.
Fig. 14 (a) Co-existence of a VGCF and a single-wall carbon nanotube (with a diameter of about 20 nm) obtained by the pyrolysis of benzene.[50] The deposition of a partial carbon layer on a carbon nanotube during the thickening process is observed. (b) Carbon nanotube (obtained by benzene decomposition) and subsequently heat treated at 2800°C, yielding the same structure as nanotubes prepared by the arc method.
The CVD synthesis method has been considered as a promising method for the large-scale production of SWNTs and also MWNTs, especially using a floating reactant technique.1-10,13,43,47-49-1 In terms of the manufacturing process, this process has been shown to be more controllable and cost efficient as compared with the arc discharge or laser vaporization methods. Fig. 15a and b exhibits a double-wall carbon nanotube and a four-wall carbon nanotube produced by a catalytic CVD meth-od.[12,49,51] For the fabrication of a carbon nanotube transistor,[52] this synthesis technique appears to be highly efficient because of the reproducibility and low cost when compared to other routes.[53,54]
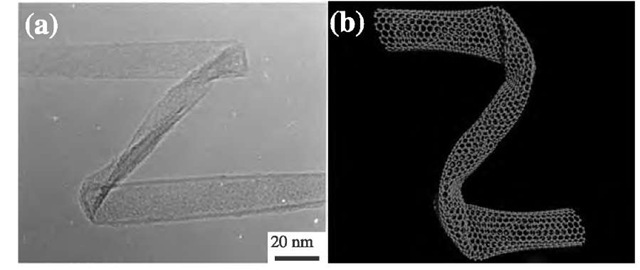
Exceptional mechanical properties of carbon nanotubes are expected, when few structural defects are present. The TEM image (Fig. 16a) and a theoretical simulated image (Fig. 16b) demonstrate that carbon nanotubes have nearly ideal mechanical properties because nanotubes do not break even after twisting, bending, or flattening. This particular behavior does not occur in carbon fibers, because they are brittle and more fragile.[50]
INTERCALATION AND DOPING BY FOREIGN MOLECULES OR ATOMS
Because of the weak van der Waals interlayer forces associated with the sp2 bonding in graphite, graphite intercalation compounds (GICs) may be formed by the insertion of layers of guest species between the layers of a graphite host material,[55,56] as shown in Fig. 17a and b. The guest species may be either atomic or molecular. In the so-called donor GICs, electrons are transferred from the donor intercalate species (such as a layer of the alkali metal potassium) into the graphite layers, thereby raising the Fermi level in the graphitic electronic states, and increasing the mobile electron concentration by two or three orders of magnitude, while leaving the intercalate layers positively charged. Conversely, for acceptor GICs, electrons are transferred to the intercalate species (which is usually molecular, such as Br2) from the graphite layers, thereby lowering the Fermi level in the graphitic electronic states and creating an equal number of positively charged hole states in the graphitic p-band. The enhanced electrical conduction in GICs (whether they are donors or acceptors) occurs predominantly in the graphene layers and as a result of the charge transfer between the intercalate and host layers. The electrical conductivity between adjacent gra-phene layers is very poor. It is noteworthy that among the GICs, Li-based GICs are widely used as anode materials in commercialized Li-ion secondary batteries for cell phones, personal computers, and electric vehicle batteries.[31]
Fig. 15 (a) Double-wall carbon nanotube and (b) a four-wall carbon nanotube produced by a catalytic CVD method.
Fig. 16 (a) HRTEM image of a distorted single-wall carbon nanotube and (b) a computer-simulated model.
Fig. 17 (a,b) Schematic model for a Li graphite intercalation compound showing the stacking of graphite layers (networks of hexagons on a sheet) and of intercalate (e.g., lithium) layers, (c) TEM image of an individual rope consisting of single-walled nanotubes aligned along the axis,[57] (d) a simulated image of a potassium-intercalated single-wall carbon nanotube rope superlattice.
Basic concepts similar to those occurring in GICs have been applied to carbon nanotubes in terms of the variation in structures, modification of electronic properties, and functionalities through the intercalation process. Namely, the bonding force between SWNT bundles or ropes (see Fig. 17c) is governed by the weak van der Waals force,[57] so that foreign species, such as atoms or molecules, can be intercalated in the van der Waals gaps,similar to graphite. Donor species, such as alkali metal atoms, can be easily intercalated into the trigonal lattice of nanotube ropes (see Fig. 17d),[58] while acceptor intercalation has proved more difficult. This result indicates the lack of 3-D structural fidelity within these nanotube ropes as it is found in 3-D bulk graphite.
Not only interstitial but also substitutional doping of foreign atoms into the carbon network of graphene sheets has also been studied widely for altering their electronic and structural properties for potential applications, such as high-temperature oxidation protection for carbon/carbon composites and as an anode material for Li-ion batteries, because, for instance, boron-doping introduces an electron acceptor level.[59] Fig. 18a shows an STM image of a 3-D surface plot and a sectional analysis of highly oriented pyrolytic graphite doped with boron.[60] Each bright area consists of boron atoms, with the highest electron density located at the center of six surrounding sites, and with medium electron density corresponding to carbon atoms. Also, the electron density distribution of a substitutional boron atom and of the surrounding six carbon atoms clearly appears in the 3-D surface plot (Fig. 18a inset). The substitutional boron atom exhibits the highest electron density in the center. Also, the substitution of boron should slightly deform the flatness of the basal plane (Fig. 18b). By restricting the measured bond length between the B-C atoms obtained from Fig. 18a, the optimized structure of a graphene sheet has been simulated. An improper torsion angle (B at the apex) is calculated to be 164° as shown in Fig. 18b, while the original plane is almost flat with an angle of 179°. For the case of multiwall carbon nanotubes, a comprehensive study of the effect of nitrogen doping was carried out using STM.[61] Fig. 19a shows an STM image of a nitrogen-doped carbon nano-tube, exhibiting both distortion and holes. The suggested structure of a CNx nanotube is shown in Fig. 19b. This type of hole might be due to pyridine-like and highly coordinated nitrogen atoms replacing carbon atoms. Substitutional doping into carbon nanotubes thus provides a useful method to modify the electronic and mechanical properties and enlarge the functionality of carbon nano-tubes even further.
Fig. 18 (a) STM image of a three-dimensional surface plot and a section analysis for boron-doped highly oriented pyrolytic graphite[60] and (b) schematic model of the top view and the side view of a boron-doped graphene sheet based on the measured dimensions of B-Cj and Cj-Cj.
Fig. 19 (a) STM image of the surface of a 20-nm CNx nanotube and (b) suggested structural model for a CNx tube.
CONCLUSION
It has been demonstrated that carbon nanotubes, consisting of graphene tubes of nanometer-size diameter, are different from conventional carbon and graphite materials, even from carbon fibers consisting of oriented planar graphene sheets. The structure of VGCFs exhibiting a carbon nanotube core can relate to both carbon nanotubes and fibers (Fig. 20).[62] The diameters range from 1 nm for nanotubes to 104 nm for carbon fibers, and VGCFs are located between the two. Bulk effects that are based on the Bernal stacking of planar graphene sheets disappear with decreasing diameter, and new quantum effects based on the thin nanotube morphology appear in the range of nanotube diameters corresponding to those of single- and multiwall carbon nanotubes. The special characteristics of carbon nanotubes in this diameter range can expand carbon science and provide new opportunities for developing innovative and exciting applications in the nano-technology field.
Fig. 20 Schematic comparisons of the diameter dimensions on a log scale for various types of fibrous carbons.

![(a) SEM image of vapor-grown carbon fibers (VGCFs),[43] (b) suggested growth mechanism of VGCFs using ultra-fine catalytic metal particles,[7] (c) very early stage of fiber growth in which the catalytic-particle is still active for promoting elongation growth. The primary fiber thus formed acts as a core for vapor-grown fibers.[43] (d) The fiber is obtained through a thickening process, such as the pyrolytic deposition of carbon layers on the primary thin fibers.[43] The encapsulated catalytic particle can be seen at the tip of the hollow core. (a) SEM image of vapor-grown carbon fibers (VGCFs),[43] (b) suggested growth mechanism of VGCFs using ultra-fine catalytic metal particles,[7] (c) very early stage of fiber growth in which the catalytic-particle is still active for promoting elongation growth. The primary fiber thus formed acts as a core for vapor-grown fibers.[43] (d) The fiber is obtained through a thickening process, such as the pyrolytic deposition of carbon layers on the primary thin fibers.[43] The encapsulated catalytic particle can be seen at the tip of the hollow core.](http://what-when-how.com/wp-content/uploads/2011/03/tmp124129_thumb1_thumb.jpg)
![(a) A vertical-type fiber synthesis system for the floating reactant method in which benzene vapor containing an organic-metallic compound such as ferrocene is introduced into a vertical-type reactor by hydrogen gas. The fibers grow while the catalytic particles are floating in the growth region of the reactor[13'43] and (b) VGCFs, obtained under controlled growth conditions, exhibit relatively homogenous diameter along the fiber length and also high purity. (a) A vertical-type fiber synthesis system for the floating reactant method in which benzene vapor containing an organic-metallic compound such as ferrocene is introduced into a vertical-type reactor by hydrogen gas. The fibers grow while the catalytic particles are floating in the growth region of the reactor[13'43] and (b) VGCFs, obtained under controlled growth conditions, exhibit relatively homogenous diameter along the fiber length and also high purity.](http://what-when-how.com/wp-content/uploads/2011/03/tmp124130_thumb_thumb.jpg)

![(a) Co-existence of a VGCF and a single-wall carbon nanotube (with a diameter of about 20 nm) obtained by the pyrolysis of benzene.[50] The deposition of a partial carbon layer on a carbon nanotube during the thickening process is observed. (b) Carbon nanotube (obtained by benzene decomposition) and subsequently heat treated at 2800°C, yielding the same structure as nanotubes prepared by the arc method. (a) Co-existence of a VGCF and a single-wall carbon nanotube (with a diameter of about 20 nm) obtained by the pyrolysis of benzene.[50] The deposition of a partial carbon layer on a carbon nanotube during the thickening process is observed. (b) Carbon nanotube (obtained by benzene decomposition) and subsequently heat treated at 2800°C, yielding the same structure as nanotubes prepared by the arc method.](http://what-when-how.com/wp-content/uploads/2011/03/tmp124132_thumb_thumb.jpg)
![(a,b) Schematic model for a Li graphite intercalation compound showing the stacking of graphite layers (networks of hexagons on a sheet) and of intercalate (e.g., lithium) layers, (c) TEM image of an individual rope consisting of single-walled nanotubes aligned along the axis,[57] (d) a simulated image of a potassium-intercalated single-wall carbon nanotube rope superlattice. (a,b) Schematic model for a Li graphite intercalation compound showing the stacking of graphite layers (networks of hexagons on a sheet) and of intercalate (e.g., lithium) layers, (c) TEM image of an individual rope consisting of single-walled nanotubes aligned along the axis,[57] (d) a simulated image of a potassium-intercalated single-wall carbon nanotube rope superlattice.](http://what-when-how.com/wp-content/uploads/2011/03/tmp124135_thumb_thumb.jpg)
![(a) STM image of a three-dimensional surface plot and a section analysis for boron-doped highly oriented pyrolytic graphite[60] and (b) schematic model of the top view and the side view of a boron-doped graphene sheet based on the measured dimensions of B-Cj and Cj-Cj. (a) STM image of a three-dimensional surface plot and a section analysis for boron-doped highly oriented pyrolytic graphite[60] and (b) schematic model of the top view and the side view of a boron-doped graphene sheet based on the measured dimensions of B-Cj and Cj-Cj.](http://what-when-how.com/wp-content/uploads/2011/03/tmp124136_thumb1_thumb.jpg)


