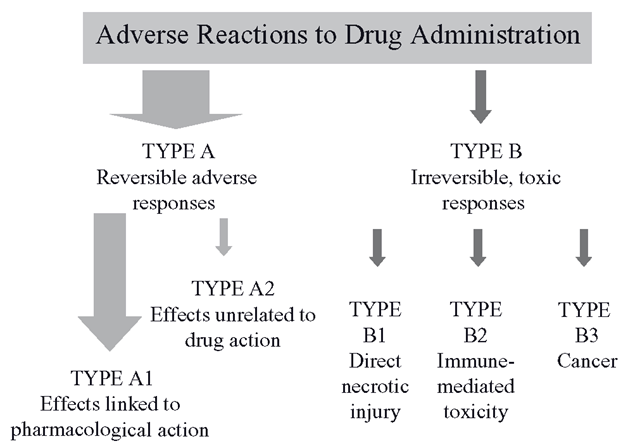
Type A describes the vast majority of adverse effects (Figure 8.1) and patients experience these in direct proportionality to the quantity of the drug and/or its metabolites in their tissues. These effects are sometimes described as ‘toxic’ but these are not usually irreversible effects. They can be resolved into two main causes, as follows.
Figure 8.1 Summary of reversible (Type A) and irreversible (Type B) drug effects
Intensification of pharmacologic effect: Type Al
This is proportional to drug concentration and happens if drug levels climb well above the therapeutic window. Anticonvulsants are membrane stabilizers, so at high concentrations they cause sedation and confusion. Similarly, high concentrations of anticoagulants cause excessively long clotting times. Some drugs may display a series of known pharmacodynamic effects at therapeutic window levels, but exert other unwelcome effects at high concentrations. Some beta- blockers at higher doses cause central effects such as nightmares. When drug levels fall, the excessive pharmacodynamic effects subside also. These adverse effects, also known generally as ‘side effects’, are mostly an intensification of the ‘main effects’, and are thought to be the cause of more than 80 per cent of patient problems with drug therapy. Many patients will experience an unpleasant concentration-dependent drug effect at some point in their lives. As we have seen in earlier topics, drug metabolism–elated changes in clearance caused by induction or inhibition of biotransformation systems can have profound and occasionally lethal effects on the patient. Other reasons for the intensification of drug effects include renal problems, overdosage and problems with dosage calculations, or being too old, too young or too sick.
Off-target toxic effects: methaemoglobin formation – Type A2
As well as off-target pharmacodynamic effects caused by parent drugs, it is possible that at least one or other of the metabolites may disrupt cellular function in a way that is unrelated to the pharmacological effects of the drug, but the disruption is reversible and predictable. This situation is again dose related, in that the more drug that is absorbed, the more is cleared through the pathway which forms a ‘problem’ metabolite. This type of adverse effect, though reversible, has the potential to make a drug almost intolerable to take from the patient’s viewpoint and can even be lethal. A good example of a reversible adverse effect is methaemoglobin formation, where the appearance of the patient’s blood becomes a passable impression of chocolate milk.
To see the link between a product of drug metabolism and an adverse drug effect, it is first necessary to understand the endogenous system involved. Haemoglobin is a molecule that normally becomes reversibly oxygenated, rather than irreversibly oxidized. Our continued existence depends on the difference between those two terms. Haemoglobin, as you know, is oxygenated to transport the gas from lung to tissues and release it where required. It is a tetramer, which means it has four protein subunits, each of which contains an iron molecule as Fe2+. The iron molecules bind the four oxygen molecules. The oxygen binding is dependent, like many enzymes, on the ability of the metal to gain and lose electrons, rather like charging and recharging a battery as mentioned previously. It is clear that a molecule like haemoglobin is potentially reactive and is going to need protection from oxidation so it can continue to function, so the erythrocyte is designed to maintain haemoglobin so that it carries oxygen and is not damaged or changed in structure. It accomplishes this with the second highest level of GSH in the body after the liver and a series of protective enzymes, more of which later. Normally, the haemoglobin iron molecules (Fe-+) bind the O2 and form superoxoferrihaem complexes (Fe-+O2-.). These dissociate at the tissues and 99 times out of 100, the Fe2+ is restored and O2 is delivered to the tissue. The one time out of 100, the oxygen retains the electron and becomes superoxide (O--.- and the Fe-+ becomes Fe-+, This is very bad, as Fe-+ will bind anything but oxygen, such as anions. So the Fe-+ means that this haemoglobin monomer is now methaemoglobin and useless for oxygen carriage. Since this is a natural consequence of transporting a reactive gas with a reactive protein, the erythrocyte carries two systems for reducing the oxidized Fe-+ to Fe2+- so restoring the function of haemoglobin. The systems are NADH diaphorase and NADPH diaphorase.Why this is the case is unknown. If it were not for NADH diaphorase, methaemoglobin formation would be around 4-6 per cent per day, more if you smoke. If you measured your methaemoglobin levels now, they would be less than 1 per cent, so the system is adequate to maintain haemoglobin in normal circumstances.
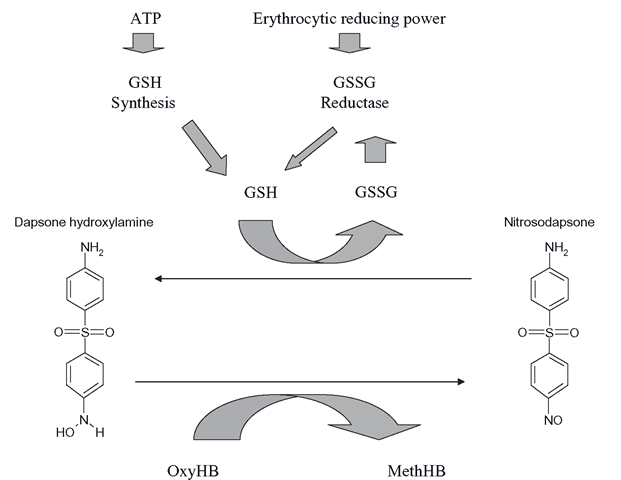
It is possible for some xenobiotics to react with haemoglobin to form methaemoglobin. Nitrites are moderately efficient at this process, but the aromatic hydroxylamines are particularly effective in forming methaemoglobin. They oxidize haemoglobin to methae-moglobin in two stages. Initially, the hydroxylamine directly reacts with oxyhaemoglobin to form methaemoglobin. This is known as a co-oxidation, as the hydroxylamine will be oxidized to a nitrosoarene. This alone would not account for the speed at which methae-moglobin can form in patients with high levels of hydroxylamine metabolites in their blood (Figure 8.2). The nitrosoarene formed by the initial reaction is then ‘helpfully’ reduced to the hydroxylamine by GSH, so allowing the hydroxylamine to oxidize another oxyhae-moglobin molecule. The resultant nitrosoarene can then be re-reduced to the hydroxylamine and oxidize another oxyhaemoglobin and so on.
The initial oxidation is thus amplified by GSH and the presence of millimolar levels of GSH is like pouring fuel on a fire. Each hydroxylamine molecule can oxidize at least four oxyhaemoglobin molecules. The result is a very rapid process, where as soon as the metabolite is produced, methaemoglobin starts forming in significant quantity, in direct proportion to the level of the metabolite released by the liver, which is of course in proportion to the drug dose.
Figure 8.2 Basic process of methaemoglobin formation in the human erythrocyte. This is repeated many times per second in a futile cycle that is limited only by the levels of GSH in the erythrocyte
Clinical consequences of methaemoglobin
Methaemoglobin is measured as a percentage of haemoglobin. However, even modest levels exert a potent effect, due to the Darling-Roughton effect. You might recall that haemoglobin is a tetramer and binds four oxygen molecules as oxyhaemoglobin. If an Fe2+ of one of the monomers is oxidized to an Fe3+, then the remaining oxygen molecules are much more tightly bound and it is harder for them to escape and oxygenate tissues. If two monomers are oxidized then it is even harder for the oxygens to be released. This means that a small percentage of methaemoglobin, say 5-10 per cent, has a greater effect than just removing 5-10 per cent of haemoglobin. The effects on the patient of methaemoglobin formation are a reflection of increasing inability of their tissues to receive enough oxygen. So at low levels, 4-6 per cent, people of fair complexions may look slightly bluish and may have a mild headache. As levels are increased to 10-15 per cent, this extends to headache, fatigue and sometimes nausea. At levels of 20-30 per cent, hospitalization is usually required, with the intensification of the previous symptoms plus tachycardia and breathing problems. Higher levels (50 per cent plus) lead to stupor and loss of consciousness, with 70 per cent plus leading to death. Methaemoglobinaemia is a drug-dependent effect that may be either acute or chronic.
Acute methaemoglobinaemia
The most dangerous situation for methaemoglobin formation can be as a result of benzo-caine usage in anaesthetized patients. The local anaesthetic is sprayed on the oropharynx prior to intubation, although not used much in the UK, it is still employed in some countries. Many formulations of the drug are around 14-20 per cent and three 1-second bursts of the spray could deliver perhaps 600mg of the drug. A number of reports have shown that methaemoglobin levels of 30 per cent or more can result in 30-40 minutes (Figure 8.3). This can be a serious problem, as blood oxygenation is usually measured by pulse oximeters and these units overestimate blood oxygenation in the presence of increasing methaemoglobin.
This problem, coupled with the lack of recognition of the visual signs of methaemo-globin in a patient on the operating table, can lead to dangerously high methaemoglobin levels without awareness of the staff. If in doubt, a CO oximeter must be used to reliably measure methaemoglobin formation. Benzocaine is unable to cause methaemoglobin itself, so it is thought that it is oxidized to a hydroxylamine, almost certainly by CYP2C9 and possibly 2C8, although CYP2E1 is thought not to be involved.
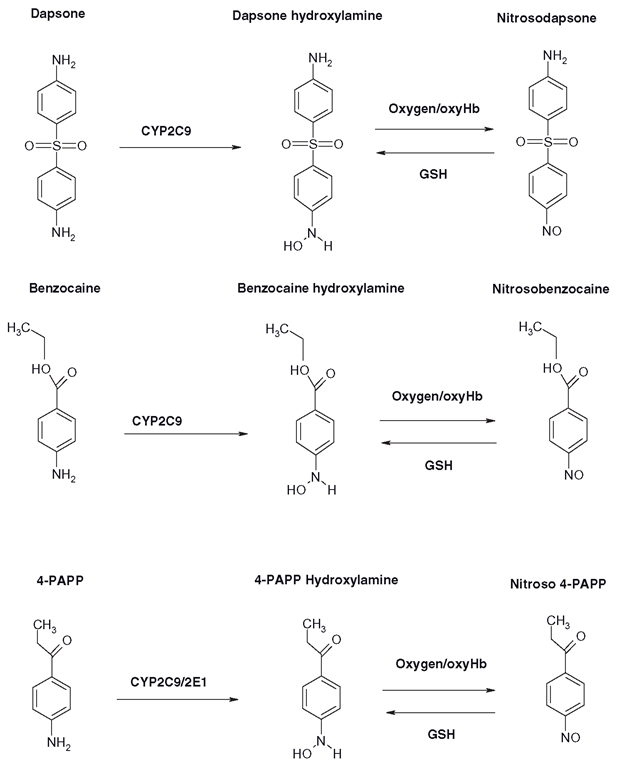
Acute methaemoglobin formation has actually been a therapeutic goal: this would appear bizarre, but cyanide is an anion, so it binds very strongly to methaemoglobin, forming cyanomethaemoglobin. The methaemoglobin has the effect of ‘vacuuming’ the cyanide out of the tissues and holding it in the blood, before a thiosulphate is given to finally facilitate the urinary excretion of the cyanide. Sodium nitrite has been employed as an antidote to cyanide poisoning; although it does not form large amounts of methaemoglobin quickly enough in cyanide overdoses where, as you can imagine, time is of the essence. A series of phenones were designed in the 1950s and 1960s that were intended to be oxidized to potent methaemoglobin formers that would be used to protect military personnel prophylactically from cyanide toxicity. How these personnel might cope with 15-20 per cent methaemoglobinaemia and maintain their combat capabilities seems difficult to imagine. However, cyanides are one of the cheapest ‘weapons of mass destruction ’ so they remain a long -term threat. The most extensively tested agent was 4-aminopropiophenone, which is also oxidized to a hydroxylamine and acts in a similar way to any aromatic hydroxylamine (Figure 8.3).
Chronic methaemoglobin formation
It is unlikely a new drug with an aromatic amine group would be approved for clinical usage today, on the grounds of toxicity. Sulphonamides retain their therapeutic place in HIV patient maintenance and their hydroxylamine metabolites are cytotoxic, and immu-notoxic; fortunately, they form negligible levels of methaemoglobin. The sulphone, dapsone, is still the mainstay of the treatment of leprosy, dermatitis herpetiformis and conditions where neutrophil migration into tissues leads to inflammatory damage.
Figure 8.3 Production of various methaemoglobin-forming species from dapsone, benzocaine and 4-aminopropiophenone (4-PAPP). In each case, the hydroxylamine is formed from the parent compound by CYP-mediated oxidation and the presence of oxygen and/or oxyhaemoglobin (oxyHb) converts the agents to nitrosoarenes, whilst erythrocytic GSH re-reduces them to their hydroxylamines within the co-oxidation cycle
Despite successful efforts to develop non-methaemoglobin forming analogues of these drugs, the market is just not large enough to sustain their development. So patients are essentially stuck with dapsone and its dose-dependent methaemoglobin formation. Dapsone is usually given at 100mg/day for leprosy and anything from 25mg/week to 400 plus mg/day for dermatitis herpetiformis. Methaemoglobin peaks at around three hours post-dosage and the patients complain of a sort of permanent ‘hangover’ effect, although tolerance varies widely. For an individual taking 100 mg/day, methaemoglobin levels may peak at 5-8 per cent, depending on their level of hydroxylamine production. The half-life of methaemo-globin reduction is just under 1 hour, so after the initial pulse, the deleterious effects of the methaemoglobin wear off within three to five hours. Many patients have to take dapsone for several years, leading to significant impact on quality of life, although coadministration of cimetidine can ameliorate this situation.Primaquine, the 8-aminoquinoline antimalarial, also forms methaemoglobin, although this is used in much shorter courses for the elimination of Plasmodium vivax, malariae and ovale (recurrent) malaria in those who are not returning to the area where they were infected. Fortunately, the drug can be effective in eliminating recurring malaria from a single 45 mg dose, so methaemoglobin formation is only a temporary problem. If longer courses are used, then methaemoglobin formation can be considerable.
Methaemoglobin as a protective process
Although potentially lethal, methaemoglobin formation is not toxicity, as the erythrocyte has two processes capable of reversing it. Once methaemoglobin is reduced to haemoglobin, it can resume its oxygen carriage function, the erythrocyte replaces its thiols and is physically undamaged. The co-oxidation process effectively ‘ -ies up ’ the hydroxylamines in the cycle and eventually releases them (via glutathione conjugates) as the parent drug. The erythrocyte thus performs a temporary detoxification of the hydroxylamine. Although methaemoglobin formation seems like an ‘own goal’- effectively the structure of the erythrocyte has been protected from the protein reactive nature of the nitroso derivative. The released parent drug will be either acetylated or reoxidized by the liver, but then it has some chance of conjugation as an N-glucuronide, so with each ‘cycle’ through the erythrocyte, the hydroxylamine fails to damage it, whilst cells such as mononuclear leucocytes are easily destroyed by nitrosoarenes.
Glucose-phosphate dehydrogenase deficiency (G-6-PD)
This condition occurs in all races, but is most common in those of Afro-Caribbean descent, with about 100 million individuals affected worldwide. This genetic polymorphism of G-6-PD is normally not an issue, except when the individuals are exposed to methaemo-globin forming drug metabolites. This enzyme supplies the majority of the reducing power of the erythrocyte and if it is poorly or nonfunctional, there is only sufficient reducing power available to supply GSH to protect erythrocytes from background levels of reactive species. As soon as the cells are exposed to high concentrations of hydroxylamines or primaquine metabolites, there is insufficient reducing power to tie up nitrosoarenes in the co-oxidation cycle and no detoxification occurs as little methaemo-globin formation happens. The nitrosoarene is then free to react with the structure of the erythrocyte. This causes the normal system of the erythrocytes’ ‘sell by date’ to be prematurely activated (they usually last 120 days) and the spleen automatically removes them from the circulation. This can happen so quickly that anaemia can result in days, with associated hepatic problems with the processing of large amounts of now waste haemoglobin. G-6-PD patients that must take either dapsone or primaquine must only receive half or less than the recommended dosage of the drugs. G-6-PD effectively converts what would be a reversible drug effect into true toxicity. If the individual is homozygous for Gilbert’s syndrome,their inadequate UGT1A1 expression will be rapidly overwhelmed by the large amounts of bilirubin formed during the erythrocytic destruction.