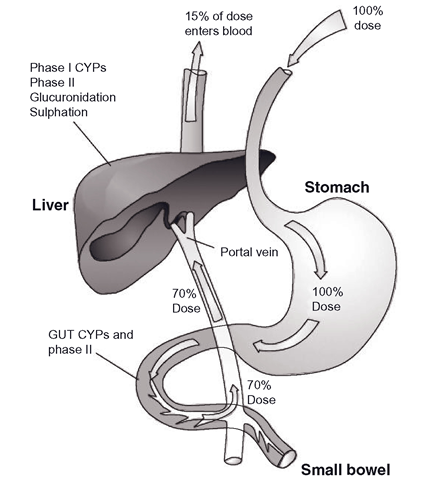
Clearance is the removal of drug from all tissues and usually the liver is seen as the major force in the clearance of drugs. However, this is an oversimplification, as other tissues can clear drugs and in the real world of a drug entering the body, the gut makes a significant contribution to clearance (Figure 1.3). To be absorbed from the gut, the drug must pass through the gut mucosal epithelial cells and enter the hepatic portal circulation, which leads directly to the liver. A drug may diffuse past the membranes of the gut epithelial cells passively, due to its relative lipophilicity or if it is more water soluble, it may require ‘help ’ from transporter systems called solute carriers. These transporters normally convey vital nutrients such as amino acids as well as drugs with similar physicochemical characteristics (like some statins). However, once in the gut epithelial cells, a drug can be pumped back out into the lumen by efflux proteins and/or metabolized by various enzymes in the gut wall cells. In the case of some drugs, this can account for a high proportion of the dose before it reaches the liver. The fraction of the original dose left then enters the liver and following hepatic extraction, most of the dose will have been inactivated. This is particularly apparent with high extraction drugs. This process, where an oral dose is metabolized by various systems, is termed ‘first pass’.
In some drugs, the vast majority of the dose is lost before it reaches the systemic circulation. The amount that actually reaches the plasma can be measured and the amount that was dosed is also known, so an equation can be produced which gives us how much enters the system. This is known as the ‘absolute bioavailability’ of the drug and is termed F. It can be defined as
Figure 1.3 The ‘first pass’ of an orally dosed highly cleared drug showing the removal of drug by the gut and liver, leading to relatively low levels of drug actually reaching the circulation
Highly extracted drugs are often stated to have a ‘poor bioavailability’. This means that the oral dose required to exert a given response is much larger than the intravenous dose. If the bioavailability is 0.2 or 20 per cent, then you might need to administer about five times the intravenous dose to see an effect orally.
Changes in clearance and plasma levels
Consider an extreme example; if the intravenous dose of a poorly bioavailable (F = 0.2), narrow TI drug X was 20 mg and the usual oral dose was 100 mg, it is clear that if the whole oral 100 mg were to reach the plasma, the patient would then have plasma levels far in excess of the normal intravenous dose, which could lead to toxicity or death. This could happen if the first pass effect was reduced or even completely prevented by factors that changed the drug’s clearance.
Similarly, if the clearance of the drug was to be accelerated, then potentially none of the 100 mg would reach the plasma at all, so causing lack of efficacy and drug failure.