PXR mediated control of CYP expression
The main NR usually associated with the control of the CYP3A series is PXR, although as mentioned above CAR is also heavily involved. PXR is also known as the SXR (steroid and xenobiotic receptor) and is classified in the nuclear receptor family as NR1I2. Although PXR is a nuclear receptor, some aspects of its behaviour resemble AhR rather than CAR.
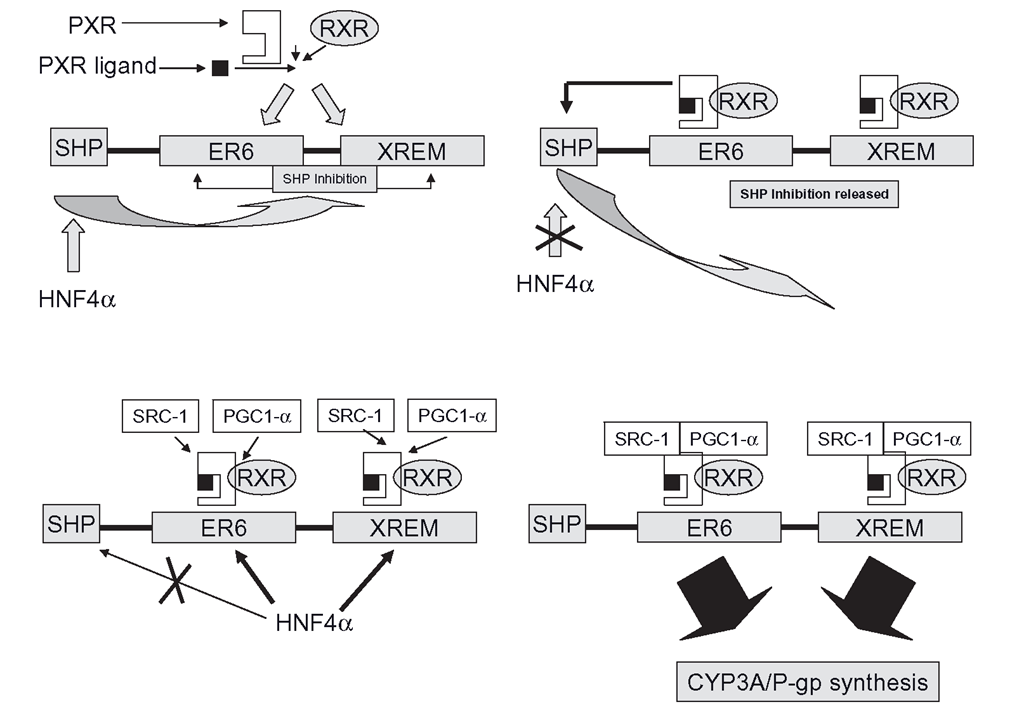
Figure 4.3 Mechanism of CYP3A induction through the pregnane X receptor (PXR) and retinoic acid receptors (RXR). HNF4a causes SHP to ‘lock’ the system. The PXR/inducer ligand binding shuts off SHP and allows HNF4a to promote the binding of the co-activators, such as SRC-1 and PGC-1, so triggering full induction.
As with AhR, PXR appears to lie dormant in the absence of any binding hormones, toxins or drugs. Unlike CAR, it has a large LBD, or binding site, which is rather quaintly termed ‘highly promiscuous’, in that it will bind a very wide range of chemical structures of all shapes and sizes. There is some degree of specificity, as rifampicin will only induce through PXR and not CAR, despite some agents which act through both NRs. This is reflected in the remarkable variation in the structures and sizes of the many clinical inducers of the CYP3A series. These include bulky antibiotics like rifampicin, several steroids as well as imidazole antifungals like clotrimazole, barbiturates and some organophosphate pesticides. It is important to note that the induction process is tailored to species, so animal studies have been less helpful in assessing the possible human enzyme-inducing properties of a novel chemical agent; rifampicin is a potent inducer of human and rabbit 3A enzymes, but it is without effect in the rat. PXR, alongside HNF4a., has an important role in controlling bile acid and cholesterol metabolism and it also part of the process where the liver displays one of its most remarkable abilities, which is to grow back after partial hepatectomy.
In terms of day-to-day operations, PXR function is a multi-stage almost simultaneous process, rather like moving off in a car (Figure 4.3). You start with the car parked with the brakes engaged, then you engage the transmission, release the brake and then press the throttle and go, more or less at the same time. Firstly, the PXR ‘Iransmission’ must be engaged: after the PXR receptors bind ligands, they recruit RXRs forming heterodimers. These then migrate to DNA and binding occurs at the CYP3A gene in two separate areas. One binding site is an ER6 in the proximal promoter of the gene and at the same time, PXR/RXR complexes also bind to another ER6 and a DR3 (Direct Repeat 3) in a second area called the XREM, or xenobiotic responsive enhancer module. The system requires both the proximal promoter and the XREM to be bound by PXR/RXR heterodimers before induction can proceed. The ‘transmission’ is engaged, but the brake must be released before the ‘throttle’ (co-activator binding) can be pressed.
Normally, CYP3A transcription is kept locked up by the presence of SHP, which performs the same role in the AhR/CYP1A induction story. The expression of SHP is controlled by none other than HNF4a and SHP’s ‘brake’ role is to prevent HNF4a from binding to the PXR/RXR bound promoter and XREM complexes, which in turn prevents the recruitment of the co-activators SRC-1 and PGC-1a , The presence of the inducer/PXR complex is thought to release the brake effectively.
The system will not fully launch transcription and translation unless HNF4a. binds the PXR/RXR complexes already bound to both the promoter and the XREM. As SHP is no longer present, HNF4a, binding then promotes recruitment of at least two co-activators, SRC-1 (also used by CAR) and PGC-1a (peroxisome proliferator-activated receptor coactivator 1). The throttle is being pressed, the brakes are off and we are in gear.
PXR system modulation
How rapidly the PXR system ‘accelerates’, that is, how potent an effect a particular inducer exerts on CYP expression seems to depend on several factors. As the process outlined above has so many steps, you would expect inducers to either compete for binding to PXR, or affect the co-repressor recruitment steps or all of the above. This is what seems to happen and given that the whole process is controlled by HNFa it is clear there is almost limitless capacity for variation in terms of the basic pre-set responsiveness of the system as well as its susceptibility to different inducers and groups of inducers. Indeed, induction in different patients has been observed to differ by more than 20-fold. Although azoles are inhibitors of CYPs, they can nevertheless modulate CYP expression through the NR system. Clotrimazole binds to PXR and promotes SRC-1 recruitment, whilst ketoconazole, known for its potent clinical CYP inhibition, can also partly block CYP3A induction as it disrupts SRC-1 binding to PXR. From a clinical point of view, the various induction processes look complex, but in some ways the process is rather like television. You can watch what is happening on the screen without entirely understanding how it works. In the same way, it is important to be aware of the drugs which cause induction and note what actually happens to drug clearance in the patient.
Interestingly, the PXR system is also implicated in drug resistance in anti-cancer therapy and is likely to be how tumour cells detect chemotherapeutic agents and respond by accelerated biotransformation and detoxification of the drugs. As PXR also controls MDR genes, which code for membrane transport systems like P-glycoprotein ,which can eject a drug as soon as it enters a cell, this nuclear receptor is vital for cancer cells to protect them from the therapeutic agent. Owing to the broadness of PXR’s binding abilities, it is thought to be a difficult receptor to block therapeutically, although it is believed sulforaphane, which is found in broccoli, is a PXR antagonist and is actually capable of down regulating CYP3A expression in human hepatocytes. Although the levels of sulforaphane required to cause this effect are in the range which is theoretically achievable through eating broccoli, this is untested in man at the time of writing. As PXR controls various transporter systems as well as CYPs, in the future, it may be possible to design agents which will prevent drug resistance in cancer therapy through selective NR antagonism.
Receptor cross-talk and CYP capabilities
Regarding CYPs and general biotransformational capability, there is a great deal of overlap in the nuclear receptor-mediated control of CYP expression. HNF4a modulates at virtually all levels simultaneously, controlling the expression and specific activities of NRs like CAR, PXR and AhR as well as expression of a large number of genes ranging from CYPs and their REDOX partners, through to conjugation systems and transporters.CAR appears to run basal ‘housekeeping’ metabolism, whilst PXR and AhR respond to both ‘emergencies’ such as the appearance of xenobiotics, as well as housekeeping in terms of bile salts and cholesterol. PXR-mediated induction, for example, is credited with the prevention of drug-induced cholestasis (cessation of bile flow) that can be caused by more than 20 drugs, including oral contraceptives, anabolic steroids and some antibiotics.
The different induction systems modulate each other also, as PXR can control AhR activity and CAR is in turn partly influenced by AhR. It is not yet established whether CAR actually modulates PXR activity. As mentioned above, CAR predominantly regulates CYP2C9 and CYP2B6, but PXR also has a role in controlling these CYPs and CAR also partly regulates CYP3A4/5, which is predominantly operated by PXR. In terms of clinical effects, many agents such as rifampicin and phenobarbitone promote the appearance of several CYPs through stimulation of both the CAR and PXR systems, whilst there are some experimental agents such as CITCO that are very specific and potent CAR stimulators, like rifampicin is to PXR. This overlap provides a ‘safety net’ to ensure that ‘threat molecules’ as well as endogenous agents that have outlived their usefulness will be controlled one way or another. It is also important to reiterate,the NR-mediated control of CYPs also extents to conjugation and transport process, providing a complete modulatory system for ensuring the detection, metabolism and excretion of hormones, drugs and toxins. There is also evidence that the drugs may influence the sensitivity of the immune system through their ability to stimulate NRs. Rifampicin’s action on PXR causes down regulation of the important immune system transcription factor NFkB, which leads to the reduction in the expression of cytokines such as TNFa. Interestingly, activation of NFkB can down regulate PXR and lead to reduced expression of CYPs. This reciprocal relationship underlines the complexity of the cross-modulation between xenobiotics and the immune system’s sensitivity and vice versa.
CYP2E1 induction
CYP2E1 is of relatively minor interest from the standpoint of drug metabolism (it oxidizes isoniazid, paracetamol and chlorzoxazone), but it is of major interest in hepatotoxin activation (paracetamol, carbon tetrachloride, thioacetamide) and carcinogen activation (N-nitrosodimethylamine, benzene, vinyl chloride and trichloroethylene). This isoform undergoes induction by apparently disparate factors like small hydrophilic molecules, such as ethanol, acetone and pyridine, as well as by systemic stresses, such as obesity, diabetes and starvation. In principle, the main points of CYP2E1 induction are now under-stood, although the details and its main physiological purpose remain to be fully explored. When animals are exposed to 2E1 inducers, functional CYP2E1 protein levels are increased up to eightfold, although the CYP2E1 mRNA levels remain the same, showing that 2E1 is not induced with a nuclear receptor regulated system like other CYPs. It is apparent that the main regulatory step of CYP2E1 is after transcription and translation are complete and the protein is actually fully assembled and shipped to the ER (or the mitochondria).
Figure 4.4 CYP2E1 induction: this CYP is not controlled by nuclear receptors and CYP enzyme is made in large quantities constantly, but in the absence of substrate, the proteosome system destroys the enzyme. The presence of the substrate effectively induces CYP2E1 by preserving it from the proteosome.
In human cellular systems, CYP2E1 remains functional for only a couple of hours before it is degraded. The presence of substrate ‘induces’ by increasing functional CYP2E1 survival time by twentyfold in comparison with substrate free cells. This suggests that CYP2E1 might be inherently structurally unstable and so is directly stabilized by its substrate, or perhaps that the substrate somehow prevents the usual rapid destruction of the CYP through another mechanism. It is thought currently that the latter explanation is more likely as CYP2E1, together with most other cellular proteins is regulated by the proteas-ome (Figure 4.4- . This structure is often likened to the cellular equivalent of a paper shredder. The proteasome is vaguely tube-shaped and contains an internal protein ‘slice ‘n’ dice’ mechanism which reduces proteins to peptides and amino acids for recycling. The proteosome works in tandem with a series of ligases which attach the protein ubiquitin to any unwanted proteins or cellular structures. The proteasome recognizes the ubiquitin-label and destroys the protein. Inhibitors of this system are actually inducers of CYP2E1 by preventing its destruction and it is thought that Hsp90 is involved. It is likely that substrate-free CYP2E1 normally contains some sort of label which the proteasome reads and automatically destroys the protein. Binding of the substrate prevents the proteosome reading the label and the protein survives as long as it binds the substrate.
It is unusual amongst normally tightly conserved and complex processes such CYP regulation, that in the absence of substrate, considerable effort is being made to produce large amounts of CYP2E1 which are more or less immediately destroyed. Why 2E1 might function in such an apparently wasteful way could lie in the specific triggers of its induction and the nature of the chemicals 2E1 is designed to oxidize. CYP2E1 is very sensitive to diet, even becoming induced by high fat/low carbohydrate intakes. Surprisingly, starvation and diabetes also promote CYP2E1 functionality. Insulin levels fall during diet restriction, starvation and in diabetes and the formation of functional 2E1 is suppressed by insulin, so these conditions promote the increase of 2E1 metabolic capability. One of the consequences of diabetes and starvation is the major shift from glucose to fatty acid/try-glyceride oxidation, of which some of the by-products are small, hydrophilic and potentially toxic ‘ketone bodies’, These agents can cause a CNS intoxicating effect which is seen in diabetics who are very hypoglycaemic, they may appear ‘drunk’ and their breath will smell as if they had been drinking. In the non-diabetic individual who is in a state of starvation, any ketone-mediated intoxication would obviously hamper the search for food, so these molecules must be cleared rapidly. The key factor here is the speed at which these compounds could accumulate – the nuclear-receptor mechanism of induction, with its time frame of days, might be too slow to cope with the accumulation of ketone bodies in starvation, so the much quicker ‘ substrate-mediated protein preservation ’ system perhaps might be rapid enough to ensure that adequate levels of CYP2E1 were present to prevent intoxication of the CNS. It is conceivable that the highly responsive control system of this CYP may also be linked with its propensity for the production of oxidative species which can promote cellular damage. This is less of a problem in the presence of high levels of a potent inducer such as ethanol, as alcoholics invariably ensure that sufficient substrate is imbibed to fully occupy the CYP and production of oxidative species might be limited. However, in the absence of such a substrate to occupy the active site CYP, a long-lived CYP2E1 might pose severe problems for a cell, producing intolerable levels of reactive oxidative species, so it is logical to degrade it quickly in the absence of an intended substrate. If this were to be the case, then the process of CYP2E1 binding and activation of oxygen should not cause enough change in the structural conformation of the CYP to stave off the remorseless proteasome shredding machine.
Non-inducible CYPs: CYP2D6
CYP2D6 is important as the main source of clearance for tricyclic antidepressants, some antipsychotics (haloperidol, risperidone), some beta-blockers and SSRIs. It is not thought to be inducible, and in cases where the clearance of a CYP2D6 substrate is accelerated in the presence of a known inducer, it is usually because CYP3A4 has been induced and this isoform is responsible for the increased clearance. CYP2D6 substrates dextromethorphan and mirtazapine clearances are markedly increased by rifampicin and carbamazepine respectively in this way. Interestingly, some studies such as those with the benzodiazepines and citalopram have shown that CYP2D6 activity increases in pregnancy. Whether this is a true induction process remains to be determined. The nearest to an induction effect seen in CYP2D6 is the expression of multiple copies of the gene which causes an ultra-fast clearance in some African and Middle Eastern ethnic groups .Although technically this could be termed an adaptive increase in CYP expression, it is not responsive to a specific drug or toxin and neither is it reversible, so it is not a true induction effect. It probably linked with diet and environment.
Reversal of induction
Anyone who has stared in disbelief at their shrunken quadriceps after a full leg plaster cast has just been removed will appreciate the main imperative which drives the reversal of induction. Just as astronauts lose the ability to walk if they spend enough time in weightless conditions without exercise, the cell will always husband valuable resources carefully and the vast increase in CYP transcription, translation and shipping to the SER is quickly curtailed in the absence of the inducer. In addition, the cell cannot afford the presence of a fully induced battery of functional CYPs without appropriate substrate, mainly because of the huge impact this biotransformational force would have on endogenous small molecules, such as hormones, seriously disrupting homeostasis. Finally, some CYPs, particularly CYP2E1, generate reactive species byproducts in the absence of substrate, so this could cause undesirable cellular oxidative stress if induction were not to be reversed.
As discussed in the sections above, the regulation of PXR, CAR and AhR all contain ‘braking systems’, which prevent CYP transcription, such as SHP. These systems act like the dead-man’s handle on a train and re-assert themselves as soon as the inducer levels fall away, although this will prevent further CYP protein assembly, the large mass of induce functional CYPs in the SER must still be prevented from functioning, by degradation and recycling.There are various systems which move finished CYP protein from the site of assembly to the SER. The CYPs must also undergo a form of quality control to ensure that they have not been mis-folded. Once they reach the SER, they must then be anchored in the lipid alongside the REDOX partners to retain functionality. These processes are not fully understood, but when induction is reversed, the shipping and quality control abruptly ceases and the pro-teasome/ubiquitin system that controls CYP2E1 shreds other CYPs, such as CYP3A4. There is also evidence that the presence of various other factors, such as the chaperone proteins like BAP31 are required to maintain the CYPs in position in the SER. Once induction ceases, these factors contribute to the release the CYPs from their SER anchor and followed by ubiquitin labeling, they are shredded and their components re-used.
Cell transport systems and induction: P-glycoprotein
Purpose, structure and function
Many endogenous agents can enter and leave cells relatively easily through passive diffusion if they are reasonably lipophilic. The more charged agents need ‘help’ to pass across membranes and the OATPs and other transporters accomplish this. Although it is necessary for cells to pump in certain required nutrients, it is equally necessary to pump others out of one cell and into another, as part of the general circulation of endogenous agents, such as vitamins, amino acids sugars and proteins. A ‘revolving door’ system like the OATPs is adequate for agents moving with a concentration gradient. It is clearly much harder to pump molecules back out of a cell against a concentration gradient. This is a bit like ejecting a few rowdy fans from a stadium into a pressing throng of people trying to enter and obviously this requires energy input. The ATP-binding cassette transporters (ABC transporters) accomplish this and there are nearly fifty of them in three main sub-families. The major system which pumps endogenous substances and xenobiotics out of cells is known as P-glycoprotein (P-gp or Pgp), which is coded for by the MDR1 gene (found on chromosome 7 in man). P-gp is a 170kDa trans membrane protein consisting of two identical halves. Each half has six column-like segments that span the cell membrane. This is the actual pump structure, whilst two nucleotide binding areas are embedded below the membrane in the cytoplasm and they bind ATP, so they are the ‘power’ pack’ of the pump.
Products of MDR genes such as P-gp possess a substrate specificity that is so wide as to be beyond promiscuity towards non-specificity; it has been described as fuzzy ’ , Some studies have suggested that substrates are likely to be lipophilic and contain a nitrogen atom that is positively charged at physiological pH ranges. In addition, substrate molecular weights are often greater than 400, have pKas greater than four, and the sum of their nitrogen and oxygen molecules are usually greater than or equal to eight. Nonsubstrates are low in nitrogen and oxygen, less than 400Mwt and a basic pKa of less than eight.
P-gp is part of the gut’s barrier function to prevent the uncontrolled entrance of xenobiotics, as the transporter effectively works in tandem with the very high levels of CYP3A that are found in the gut (three times as much as in the liver in humans). If P-gp repeatedly pumps an agent out, it has more chance of meeting a CYP on its next entry. The system also has an element of insurance, as inhibitors of CYPs such as grapefruit juice do not necessarily always inhibit P-gp, so some residual barrier function remains as seven times as much P-gp is found in the apical areas of enterocytes compared with hepatocytes. The presence of P-gp can certainly retard drug absorption, without actually preventing it.
P-gp induction: clinical effects
P- gp is under the control of both PXR and CAR and potent inducers of CYP3A and CYP2C9 are well known to induce it. Rifampicin, St John’ s Wort and carbamazepine cause large increases in gut P-gp and often CYP3A substrates are also transported by P-gp, causing a combined effect of reduced cellular entry and accelerated clearance of drug molecules that do enter the gut. However, the clinical consequences of P-gp induction are not confined to CYP substrates. There are many drugs that are substrates of P-gp but are not metabolized by CYPs. Rifampicin can cause marked reductions in these substrate bioavailabilities through this mechanism without affecting their oxidative metabolism. The effects on digoxin are particularly serious, as it has such a narrow TI (0.5-3ng/mL) and levels that exceed 3.5 ng/mL increase the risk of patient death. The therapeutic monitoring of this valuable but toxic drug has been revised to improve its safety and it is now recommended that the therapeutic window for this agent should be between 0.5 -0.8 ng/mL. Clearly any drastic changes in bioavailability will make a strong impact on the patient, either through loss of efficacy or toxicity. Rifampicin is known to reduce the bioavailability of digoxin and St John’s Wort’s effects on cyclosporine (causing tissue rejection, see below) are at least partly due to its potent induction of P-gp through PXR. It is likely that these agents will also have the same impact on other anti-Iissue rejection drugs such as tacrolimus. These agents are very difficult to manage clinically, as they can exert toxicity in all the major organs, including the transplanted organ or graft itself. Tacrolimus, for example, has a terminal half-life of the best part of four days and a plasma level of 30 ng/ mL is considered the upper limit before toxicity ensues. Although cyclosporine has been reported to have a slight inductive effect on P- gp, some cellular studies suggest that tacrolimus may decrease P-gp expression and function, so it may potentiate the effects of other drugs in terms of tissue penetration.
It has long been known that as many as 40 per cent of epileptics show resistance to drug therapy with AEDs. It has been proposed that P-gp or other ABC transporters ‘protect’ brain tissues from these drugs. It has been controversial as to whether some or all of the AEDs are P-gp substrates in the human brain. Recent work suggests that phe-nobarbitone, phenytoin may be P-gp substrates, lamotrigine and levetiracetam also, but not carbamazepine. Whilst results from -n vitro work may be dependent on the type of assays used, it does appear that many of the AEDs could be P-gp substrates. If they are, then as many AEDs are inducers of CYPs, particularly CYP3A4, they should induce P-gp and MRPs in all tissues as well as the brain. This process should theoretically promote over time the exclusion of the AEDs from the very neural tissues they are designed to treat. This is extremely difficult to prove and is hard to investigate as patients are often taking many other drugs and have different ethnic backgrounds. Apparently, patients who had a very high number of seizures before treatment were more likely to have breakthrough seizures in the presence of an AED, so it is possible that ABC transporter-mediated issues may only be a component of the larger pharmacodynamic picture of drug resistant epilepsy.
P-gp and cancer
The P-gp system is found in virtually every species and is the subject of intense interest, as bacteria, protozoa and human cancerous cells all use it to protect themselves from potential toxic agents by detecting the toxin. This usually occurs through a nuclear receptor such as PXR, which induces P-gp to pump the drug out, be it an antibiotic or an alkylating agent. Indeed, the MDR1 gene that codes for P-gp actually stands for multi-drug resistance gene 1. If P-gp appears in sufficient quantity to clear the agent as fast as it enters, even potent cytotoxins will exert little or no effect, rather like wafting your hand through a flame quickly so it doesn’- burn. Unfortunately, around half of all anti-cancer drugs are substrates for ABC -type transporters, which includes other pumps such as the MRPs.It is possible that resistance to anticancer agents occurs through their binding to NRs such as PXR, as well as to other NR systems, although chemotherapeutic resistance seems to emerge more slowly than the timeframe of 2-4 weeks that the classical nuclear receptor induction process involves. It is possible that P-gp and its fellow pump systems are linked to other cellular receptor systems; this is because many anticancer drugs are not inducers of CYPs but do eventually induce local P-gp in the tumour target.
As discussed later in this topic, cancer patients take large numbers of supplements and drugs related to the stress of their condition and this can have a serious impact on their therapy. St John’s Wort accelerates the clearance of anticancer agents such as irinote-can and the tyrosine kinase inhibitor (TKI) imatinib and the Wort’s induction of P-gp-mediated drug resistance at the cellular level may also be a critical factor in treatment outcome. Hence, not only do PXR inducers like St John’s Wort accelerate drug oxidative metabolism, but their induction of P-gp is quite likely to blunt tumour penetration of what parent anti-cancer drug does enter the body and probably the metabolites also, thus effectively promoting resistance. P-gp induction’s effect on pro-drugs metabolized by CYPs is likely to be more complex, but similarly deleterious.