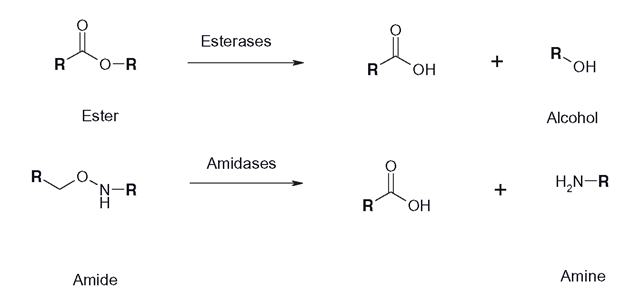
There are a number of drugs that possess ester or amide linkages and these are vulnerable to the activity of esterases and amidases. Esterases are sometimes known as carboxyleste-rases and can overlap in activity with amidases (Figure 6.12). These enzymes clear procaine, acetyl salicylate and chloramphenicol, although genetic polymorphisms can prolong the clinical effects of a number of neuromuscular blocking drugs (succinylcholine, atra-curium mivacurium) that are hydrolyzed by butyrylcholinesterase.There are many esterase enzymes in various tissues and the plasma and they are responsible for the rapid clearance of cocaine and heroin.The liver and kidney enzymes can hydrolyze the drugs listed above as well as several organophos-phate and carbamate insecticides, as well as some herbicides. All the esterases, which include neural acetylcholinesterase and butylcholinesterases, operate in a fairly similar manner, using an anionic and esteratic site to bind the substrate and a serine hydroxyl group to catalyze the hydrolysis of the ester linkage. Once an amidase has hydrolyzed an amide, if an aromatic amine is released, the subsequent oxidative N-hydroxylation of the amine can cause systemic toxicity.
Figure 6.12 Esterase and amidase reaction sequences