Introduction
The hemp plant Cannabis sativa is the main source of cannabinoids, a group of around 60 terpene psychoactive agents which are probably the most commonly used illicit substances for recreational purposes. In the plant itself, the richest source of the cannabinoids is the resin removed from the leaves of the female plant. The most potent of the cannabi-noids is Δ-9 tetrahydrocannabinol (Δ-9 THC; Figure B.10), although Δ-8 THC is as psychoactive and is chemically more stable. Cannabinoids are usually prepared from the dried flowering tops and leaves (marijuana, up to 5 per cent Δ-9 THC), the resin itself combined with the flowers (hashish, up to 25 per cent Δ-9 THC) and the industrial strength version, hash oil. This is resin that has been extracted and purified with solvents and concentrated and it can exceed 70 per cent in Δ-9 THC content. As cannabis is rather bulky to smuggle and law-enforcement interception rates have improved, it was found that crossing Cannabis sativa with its less palatable cousin Cannabis indica happily yielded a hardier, smaller, more potent and profitable version known as ‘skunk’ which can be grown unobtrusively in quantity in a suburban house next door to you. Skunk can have three times the Δ-9 THC content compared with imported cannabis. A great deal of illicit research and development has led to the use of high intensity lighting which induces the plants to form more resin which is part of the effort to produce greater Δ-9 THC content. Indeed, the growing process is a race against time before the power company notifies local law-enforcement over the stadium-sized electricity bill.
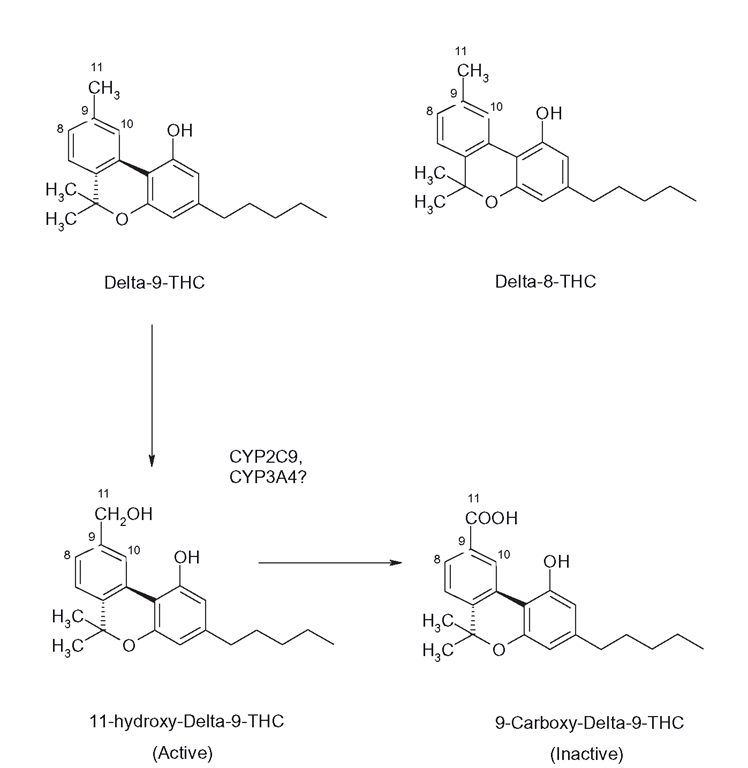
Figure B.10 The main active constituents of Cannabis sativa, Δ-8 and Δ-9 THC and the clearance of Δ-9 THC to its hydroxyl carboxy products by CYPs 2C9 and possibly CYP3A4
The cannabinoids are quite potent and a dose of less than 10 mg is required for the standard ‘high’ effects, although they also have stimulant, sedative and even hallucinogenic properties. It is now clear that these agents they have more in common with opiates than other drugs of abuse such as cocaine, in that there are specific cannabinoid receptors known as CB1 and CB2. Endogenous agonists are anandamide and 2-AG, which are made on demand and then hydrolyzed by an enzyme known as FAAH, which is of interest as a possible drug target to potentiate the effects of the endogenous molecules. It seems that cannabinoid receptors act by inhibiting the release of neurotransmitters responsible for anxiety, like glutamate and GABA. THCs are agonists, whilst other agents in cannabis, such as cannabidiol (CBD) act as antagonists. Essentially, the mixture of psychotropic (mainly THC mediated) and anxiolytic (CBD) effects will vary according to the contents of the respective agents, route of administration and dose. Thus cannabis consumption leads to a complex mixture of effects targeted on areas of the brain rich in the CB1 receptors, such as those associated with cognition, memory and movement coordination. CB1 receptor activation by higher doses is noticeably different from lower doses and this may be linked the intense paranoia and psychosis, linked with heavy use, which can lead to users being ‘sectioned’ under the Mental Health Act. This process is exacerbated by the accumulation of active THC derivatives and skunk’s low CBD levels. The CB1 receptors are also found in the periphery alongside CB2 receptors and their endogenous functions are wide–anging and not entirely understood. Several synthetic agonists and antagonists have been synthesized. The first CB1 antagonist, rimonabant, was briefly introduced for treatment of obesity, but was withdrawn in 2008 due to CNS problems, which suggests that we have some way to go to understand the full profile of cannabinoid receptor function. In contrast, Sativex, a mouth spray that consists of equal proportions of CBD and Δ-9 THC, has shown promise in relief of pain and spasticity associated with multiple sclerosis and was approved in Canada in 2005. It is available at the time of writing in the UK on a ‘named patient basis’.
It seems the main reason that cannabinoids are not very toxic is that unlike, say, opiate receptors, they are not found in vital neural areas that control respiration or heart rate. It is possible that more subtypes of cannabinoid receptors will be found and the full medicinal potential of these receptors will emerge in years to come. For instance, it is believed that the anti-angiogenic and antimetastatic properties of cannabinoids may be exploited in future anti-neoplastic agents.
Metabolism
THC-derivative clearance is extensive, with dozens of metabolites formed; indeed, only small amounts of parent Δ-9 THC appear in urine. The half-life of Δ – 9 THC in blood is around 20 hours, although the effects from smoking appear within 5-10 minutes. When cannabis products are smoked, Δ-9 THC is cleared within a few minutes to 9-carboxy Δ-9 THC (Figure B.10), which is pharmacologically inactive and is the major urinary metabolite. This metabolite is used to monitor cannabis usage in drug -testing protocols. It is unclear as to which CYP performs this function, although it is believed that CYP3A4 may form oxo-metabolites from 7- and 8-hydroxy derivatives of Δ-8 THC, which retain activity, although 2C9, 1A1 and 1A2 were also involved. It is interesting that school friends of mine who used the drug in the late 1970s insisted that it was far better consumed orally than smoked and that the effects were more pleasant and longer in duration. Indeed, it is now known that this is the case, as oral dosage promotes the formation of 11-hydroxy Δ-9 THC which is not only more potent than Δ-9 THC, but enters the brain more quickly. There is some evidence that CYP2C9 catalyses this reaction. It is possible that chronic use leads to induction of THC metabolism, but the metabolites are still very lipophilic and accumulate in fatty areas. After around a week, more than one-third of the dose is still in the user. The metabolites’ oil solubility means that they are consequently difficult to clear into urine and complete excretion of the various metabolites usually occurs in faeces and can take months after high doses. Hence, the consumption of small quantities can lead to the failure of a random drug test several weeks later.
Carcinogenic activity of cannabis products
It is hotly debated by various groups as to whether cannabis smoking does lead to increased risks of lung cancer. Logically, it would appear to be very hard to separate the effects of tobacco, a known carcinogen, from the cannabis itself, as they are more often than not co- administered. There is evidence both for and against the possible carcinogenic properties of cannabis; in animal cell-üne studies, cannabis was shown to be capable of inducing CYP1A1 activity, thought to be a key factor in lung carcinogenesis. Indeed, Δ-9 THC was shown to act through the Ah receptor system in classical fashion. However, Δ – 9 THC was also shown to competitively inhibit the induced CYP1A1; what this might mean for possible carcinogenesis over many years of use is difficult to extrapolate. If Δ-9 THC exposure was fairly constant, then CYP1A1 levels would be maintained in accordance with exposure. It has been suggested that when CYP1A1 is ‘ -dle – - i.e. not oxidizing substrates, it ‘teaks’ reactive species that lead to DNA damage. Since THC derivatives are so persistent, it is possible that there may not be much time when the isoform is ‘idle’, so the THC might restrict reactive species formation. Clearly, arguments could be made in the opposite direction and on balance it is unlikely that occasional use would be carcinogenic, just as occasional use of tobacco is much less risky than heavy use. That the issue is not resolved after more than 25 years of study indicates that confounding factors such as the lack of filters on cannabis cigarettes, the variability of dose, the difficulty in estimating concurrent tobacco exposure and the effects of THCs on the immune system may well make it impossible to prove conclusively whether marijuana use is carcinogenic. It is, however, not unreasonable to suggest that heavy use, which is effectively abuse, will increase the risk of lung neoplasms. Some have suggested (hopefully ironically) that the more potent forms such as skunk would be less carcinogenic, on the grounds that users will limit their consumption and thus potentially their carcinogenic exposure.