The saturated bonds of straight chain aliphatic molecules are very stable; indeed, they can be even harder to break into from the thermodynamic point of view than aromatic rings, whilst molecules with unsaturated bonds are the easiest to oxidize. Straight chain aliphatic molecules are easier to oxidize if they have an aromatic side chain. Alkyl derivatives are generally oxidized by the routes briefly described below.
Saturated alkyl groups
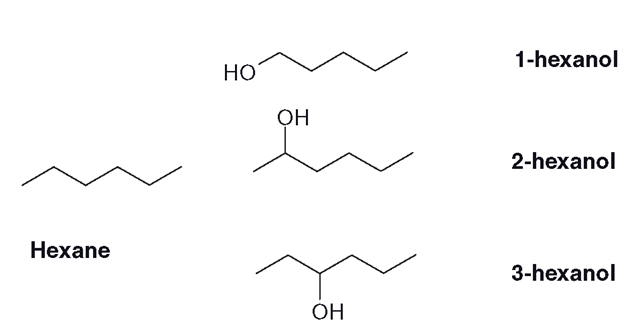
The oxidation of a saturated alkyl group can lead to the alcohol being inserted in more than one position (Figure 3.10). The ‘end’ carbon group of the molecule is sometimes called the ‘omega’ group and the oxidation can result in this group being turned into an alcohol (omega oxidation) or alternatively, the penultimate group (omega minus one).
Figure 3.10 Omega and omega minus one carbon oxidation of aliphatic saturated (single bond) hydrocarbons by CYP isoforms
During the oxidation of saturated molecules (Figure 3.10, the CYP will operate as described in section 3.7, abstracting hydrogen and causing the carbon molecule to form a radical. The carbon radical and the hydroxyl group then react to form the alcohol. Even though alkanes like hexane are very simple structures, they can be metabolized to a large number of derivatives (see section 3.11.3, ‘Pathways of alkyl metabolism’).
As well as the formation of alcohols, CYP isoforms can desaturate carbon-carbon double bonds to single unsaturated bonds (Figure 3.11). This process can occur alongside alcohol formation and is a good example of a CYP-mediated process that leads to quite considerable rearrangement of the molecule’s structure. The first hydrogen is abstracted by the CYP isoform and may leave the FeO3 + complex, allowing it to grab a second hydrogen atom. The highly unstable adjacent carbon radicals rearrange to form an unsaturated product. The two hydrogens and the single oxygen atom that the CYP enzyme used to accomplish this effect form water.
Figure 3.11 Formation of unsaturated bonds from a saturated starting point
Unsaturated alkyl groups
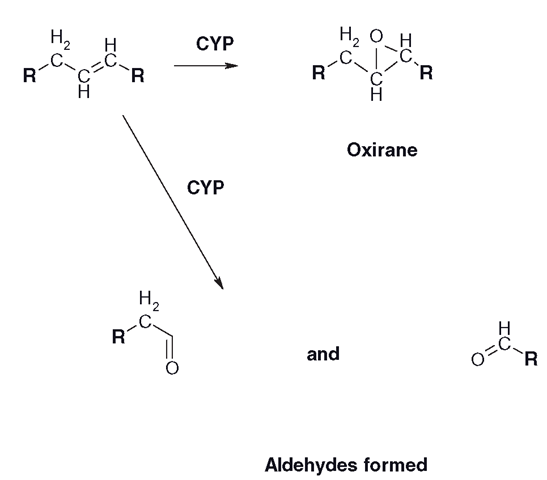
Unsaturated or double bonds are more electron-rich than saturated bonds, and as mentioned earlier, this makes them easier to oxidize and several possible products can be formed (Figure 3.12). These include an epoxide called an ‘oxirane’, as well as two carbonyl derivatives, or aldehydes, which can split the molecule.
Pathways of alkyl metabolism
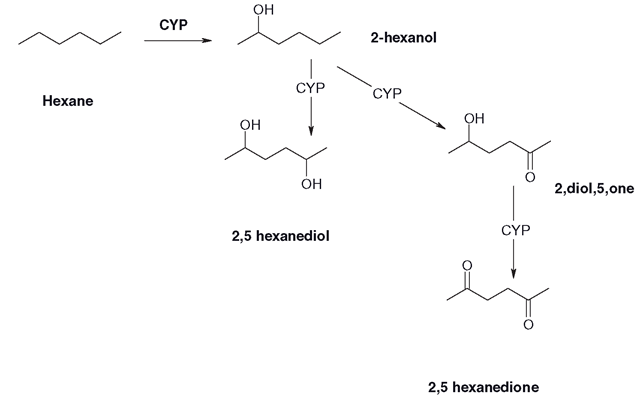
A good example of where these pathways can lead is the complex metabolism of an otherwise apparently simple molecule, hexane (Figure 3.13). This hydrocarbon was once used as a volatile component of several adhesive mixtures, which were extensively applied in the leather and shoe industries. If you want an adhesive or paint to dry or cure quickly, the volatility of the carrier solvent is crucial. This makes the adhesive or paint easier to use in a mass production setting. However, it was gradually realized that many people who used hexane-based adhesives in the leather industry were suffering from damage to the peripheral nervous system, known as peripheral neuropathy. This was a progressive effect and was traced to the hexane itself. In humans, hexane is cleared at first to several hexanols, which is logical, as a volatile, water-insoluble and highly lipophilic agent capable of causing intoxication will be a strong candidate for rapid clearance to an albeit only slightly water- soluble alcohol.
Figure 3.12 Metabolism of unsaturated alkyl groups
Figure 3.13 Oxidation of hexane to hexanols
The 2-hexanol derivative undergoes further oxidative metabolism, initially to a diol, the 2,5 derivative (Figure 3.14), which can undergo further CYP isoform-mediated (probably by 2E1) oxidation to a di-ketone which is the 2,5 hexanedione derivative. This compound is unusual in that it is a specific neurotoxin. It interferes with microtubule formation in neural fibres, causing gradual loss of neural function. It is also cytotoxic to neuronal cells and its relatives, the 2,3 and 3,4 diones, are cytotoxic to cell cultures. Consequently, n-hexane is banned from use in adhesives and should only be used where the fumes cannot be inhaled. The 2,3 and 3,4 hexanediones are used as food colourings and flavourings, although it is unlikely they can be formed in human liver. Several isomers of hexane have been used as substitutes for hexane, although the potential neurotoxicity of adhesives that use volatile alkanes should never be underestimated.
Figure 3.14 Formation of the neurotoxin 2,5 hexanedione by CYP oxidations