Non-replicating HFV
The second generation FV vectors were non-replicating HFV, lacking portions of the structure gene. The recombinant foamy virus vectors (pFGPSN and pFGPMAP) were constructed by replacing a portion of the env gene with the neomycin phosphotransferase gene under the control of the SV40 early promoter and the human placental alkaline phosphatase gene under the control of the MLV LTR promoter, respectively (David & Russell, 1996). These vectors were capable of transducing diverse cells (David & Russell, 1996), including human fibroblasts, COS-7 cells, Vero cells, Cf2Th cells, etc. Furthermore, these vectors transduced cells from different species, including steer, sheep, dog, cat, rat and hamster and at different stages of the cell cycle, thus suggesting that HFV could transduce dividing and non-dividing cells.
Bel-1-independent HFV
The third generation FV vectors were Bel-1-independent HFV. This foamy virus vector system contained relevant packaging plasmid (pCGPES) and vector plasmids (pCGPMAPDBel and pCGPMscvF) (Vassilopoulos et al., 2001). In this vector, a constitutive CMV/HFV fusion promoter derived from the cytomegalovirus (CMV) promoter replaced the HFV LTR U3 region sequences (Trobridge & Russell, 1998). The gag, pol, and env were placed under control of an SV40 polyadenylation site (SV pA) in the packaging plasmid pCPGES, and the reporter gene, AP or GFP, was expressed respectively by internal MLV LTR or murine stem cell virus (MSCV) promoters (Vassilopoulos et al., 2001). Thus, the gag and pol genes were not expressed in transduced cells after reverse transcription. Researchers demonstrated that the 5′ portion of gag and 3′ portion of pol were critical for viral product (Heinkelein et al., 1998; Erlwein et al., 1998). Thus, it may be possible for foamy virus vectors to contain fewer viral sequences in the future.
Human foamy virus hybrid vector
To improve the viral titer, adenovirus and human foamy virus hybrid vector systems (pFAD-2 and pFAD-7) were developed (Picard-Maureau et al., 2004). An infectious extracellular particle could be produced by pFAD-2 containing the PFV gene. The pFAD-7 lacked the PFV env gene required for viral capsid export and could therefore only undergo an intracellular replication cycle. Both of these FV vectors were controlled by the tetracycline-regulatable system. The other adenoviral/PFV hybrid vector was generated from three different adenoviruses and achieved gene transfer in vitro (Russell et al., 2004). These vectors separately expressed the PFV structural genes gag and pol (Ad-GagPolDPacI), the PFV structural gene env (Ad-Env), and the transgene (Ad-MD9). After co-transduction by the three adenoviruses, release of recombinant PFV was generated, and a titer of up to 103 vector particles/ml was achieved.
It has been shown that these hybrid vectors can effectively improve the titer and integrate into host genome. Additional foamy virus hybrid vectors have been developed, and more research needs to be undertaken to verify their effectiveness and safety.
FVs derived from other species
In addition to the prototypic PFV (Trobridge et al., 1998; Heinkelein et al., 1998; Trobridge et al., 2002; Schmidt et al., 1995; Nestler et al., 1997; Heinkelein et al., 2002), a variety of FV vectors have been developed, including simian foamy virus type 1 (SFV-1, macaque) (Wu et al., 1998; Park et al., 2002) and feline foamy virus (FFV) (Bastone et al., 2006; Bastone et al., 2007). A series of vectors and helper plasmids derived from SFV-1 have been constructed, and the minimum vector sequence required for efficient gene transduction has been established. This minimum vector contained the 5′ untranslated region to the first 637 nucleotides of the gag, 596 nucleotides of pol, while the 3′ LTR removed 1131 nucleotides. Therefore, with different packaging plasmids and helper plasmids, this vector can carry an 8930 base-size heterologous DNA fragment.
The advantages of FV
Research groups have designed a novel type of vehicle based on FVs for use as gene delivery vectors. FVs are classified in the subfamily Spumaretroviridae (Khan et al., 2009). FVs have been reported to be prepotent in relation to other retroviral subfamilies, such as HIV and HTLV, in terms of safety and efficiency. FVs have many advantages that allow them to transduce target cells for treating nervous system disorders. The advantages of functional gene transfer vectors derived from foamy viruses are as follows.
a. FVs have a wide range of hosts and can infect a variety of tissues and cells (Heneine et al., 1998; Russell & Miller, 1996). They were first found in mammalian species, while humans can be infected through occupational and non-occupational exposure to infected animals and their tissues, blood or body fluids (Khan et al., 2009). The feature of a broad host tropism makes FVs suitable for wide use in gene therapy.
b. Most retroviral vectors derived from FVs are nonpathogenic to humans (Caprariello et al., 2009). There is not enough evidence indicating the ability of foamy viruses to be transmitted between populations (Liu et al., 2005; Mergia et al., 2001). Furthermore, long-term studies of animal care workers and experimental researchers infected by foamy viruses have failed to show negative consequences (Mergia et al., 2001; Saib et al., 1997). Thus, there is little or no risk of developing malignancies in patients treated with gene therapy by FV vectors.
c. The size of the foamy virus genome is the largest amongst all retroviruses (Flugel et al., 1991; Trobridge et al., 2002). Thus, it is able to transport large fragments of foreign genetic material into cells. As therapeutic genes can be too large to be delivered by common retroviral vectors, the ability of FV vectors to package foreign genes is significant. FVs can be used as gene therapy vectors for the diseases that are caused by the loss of large fragments of functional genes.
d. FVs possess a dual-promoter, which makes them unique. Whereas all retroviruses have a promoter in their U3 region of the long terminal repeat sequence, FVs have an internal promoter that is located at the end of the env (Liu et al., 2005; Lochelt et al., 1993). The two promoters co-regulate the expression of therapeutic genes at both temporal and spatial levels in target cells.
e. FVs have a distinct integration pattern compared with other retroviral vectors. Bauer et al. have studied these patterns, and unlike gammaretroviral and lentiviral vectors, there seems to be an inverse correlation between gene density and integration frequency. Furthermore, FV vectors integrated significantly fewer times near oncogenes, as demonstrated using integration-site and statistical analysis (Bauer et al., 2008). The unique integration pattern indicates that FVs prefer inserting into non-transcribed areas of the chromosome, which promotes the development of FV vectors in gene therapy.
f. Recently, researchers have found that the number of integration sites in a cell gradually increased over several weeks following viral infection. Most of the additional integration sites appeared in regions of low gene density (Bauer et al., 2008). This may be, to some extent, why FV vectors are safer and have a higher expression as well as long-term efficacy.
g. FVs particles are simple and convenient to obtain for basic medical research and clinical trials. FVs are stable enough to be concentrated by ultracentrifugation and maintain the ability to introduce and express genes in target cells (Josephson et al., 2004).
These unique characteristics of FVs make them suitable to be used as foreign gene delivery systems in treating genetic diseases, especially nervous disorders. However, low viral titer limits the application of FV vectors to clinical treatments. Furthermore, it is uncertain whether a mutation could occur after integration, which would be a serious threat to the health and survival of individuals undergoing gene therapy treatment.
Transduction of neural and other cells by FV vectors
Transduction of a variety of cells derived from diverse species
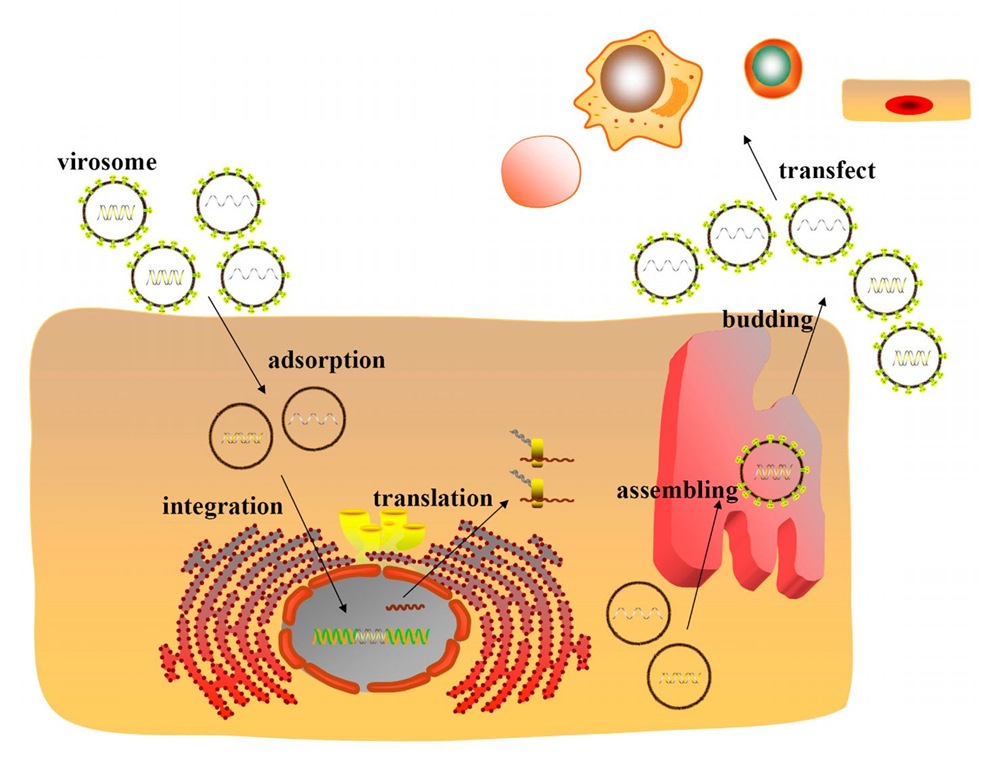
FV vectors can transduce diverse human cells (David & Russell, 1996), including human fibroblasts, 293 cells and COS-7 cells. FV vectors can also transduce cells derived from other species, such as, Vero and Cf2Th cells. These cells can be transduced by FV vectors at different stages (M phase or stationary phase) of the cell cycle (Figure 3).
Fig. 3. The cell model transduced by FVs. Infectious viral particles enter cells and concentrate around the centrosomes. Under the help of centrosomes, the PIC enter the nucleus. After integration, the viral genes are expressed, and the viral proteins are synthesized. The viral particles are assembled in internal membranes, and released by budding.
Transduction of growth-arrested cells
Research has demonstrated that FV vectors can transduce MRC5 cells and human fibroblasts that have been arrested using aphidicolin (G1/S phase) (Saib et al., 1997; Trobridge et al., 2004), although the integration pattern (integration or non-integration) remains unclear.
Transduction of neural cells and brain tissues
NT2.N neurons, a postmitotic human neuronal cell line, can be transduced by simian foamy virus-1 (SFV-1) vectors (Mergia et al., 2001). Neural cells, cultured astrocytes, cultured rat hippocampal and dorsal root ganglia neurons are also transducible by FV vectors (Liu et al., 2005; Liu et al., 2007; Liu et al., 2008). Brain tissues can be transduced by FV vectors in a rat model (Caprariello et al., 2009).
Possible mechanisms for the transduction of neural cells and other growth-arrested cells
Although the underlying mechanism of foamy viral transduction in neural and nondividing cell is unclear, some clues can be acquired from the unique life cycle of FVs (Figure 3).
The viral structure of foamy viruses is special. FVs possess two coordinated promoters (Liu et al., 2005; Lochelt et al., 1993): one is located in the U3 region of the LTR, while the other, termed IP, is located at the end of the env gene. With the largest genome among all retroviruses (11,021 bp), FV vectors can carry a large transgene cassette (9.2 kb) (Flugel et al., 1991; Trobridge et al., 2002.)
Foamy viral reverse transcription, integration, transcription and packaging processes are also distinct. The reverse transcription during viral particle formation produces 20% of viral particles containing the full-length viral cDNA genome (Mergia et al., 2001), which is conducive to the formation of the PIC. The Tas (transactivator of spumavirus) plays a critical role in foamy viral replication and transcription. The pol gene of FVs is directly spliced from the mRNA, which is unique to FVs (Yu et al., 1996; Enssle et al., 1996). Therefore, the protease reverse-transcriptase-integrase proteins, derived from the Pol, are activated early and may facilitate the formation of the preintegration complex (PIC). As an element of the PIC, the Gag protein is cleaved into a mature product near the C-terminus. The entrance of the PIC into the nucleus utilizes the centrioles. The frequency of foamy viral integration is inversely correlated with gene density, with foamy virus preferring to integrate into regions of low gene density (Bauer et al., 2008).
Application of FV vectors in neurological disorders and other diseases
It has been reported that 2 of 11 children who received gene therapy developed leukemia due to the use of retroviral vectors, which may lead to the insertional activation of nearby oncogenes (Marshall, 2002; Marshall, 2003). In contrast, foamy virus is a safe and promising vector system for gene transfer into various cell types, including neurocytes. The potential applications of FV vectors for gene therapy are summarized below and in Table 1.
|
Virus |
Diseases |
Transgenes |
References |
|
HFV |
Parkinson’s disease |
GAD gene |
Liu et al. 2007 |
|
HFV |
Neuropathic pain |
GAD gene |
Liu et al. 2008 |
|
FV |
Lukocyte adhesion deficiency |
MSCV- CD18 |
Bauer et al. 2008 |
|
FV |
Lukocyte adhesion deficiency |
PGK-CD18 |
Bauer et al. 2011 |
|
PFV |
Gioblastoma xenograft model |
FOV-7/pnp, FOV-7/ntr, FOV-7/tk suicide gene |
Heinkelein et al. 2005 |
|
HFV |
Cancer |
Interleukin-24 |
Chen et al. 2010 |
|
HFV |
HIV infection |
Interferon-tau |
Fujii et al.2004 |
|
SFV-1 |
SIV infection |
R2 siRNA |
Park et al. 2005 |
|
FV |
HIV infection |
RevM10, Sh1 and maC46 |
Taylor et al.2008 |
|
HFV |
Hpatitis B |
siRNA |
Sun et al. 2007 |
|
FV |
Cronic granulomatous disease |
gp91phox |
Chatziandreou et al. 2011 |
Table 1. Foamy Virus Vectors used in Neurological Disorders and other diseases
Parkinson’s disease
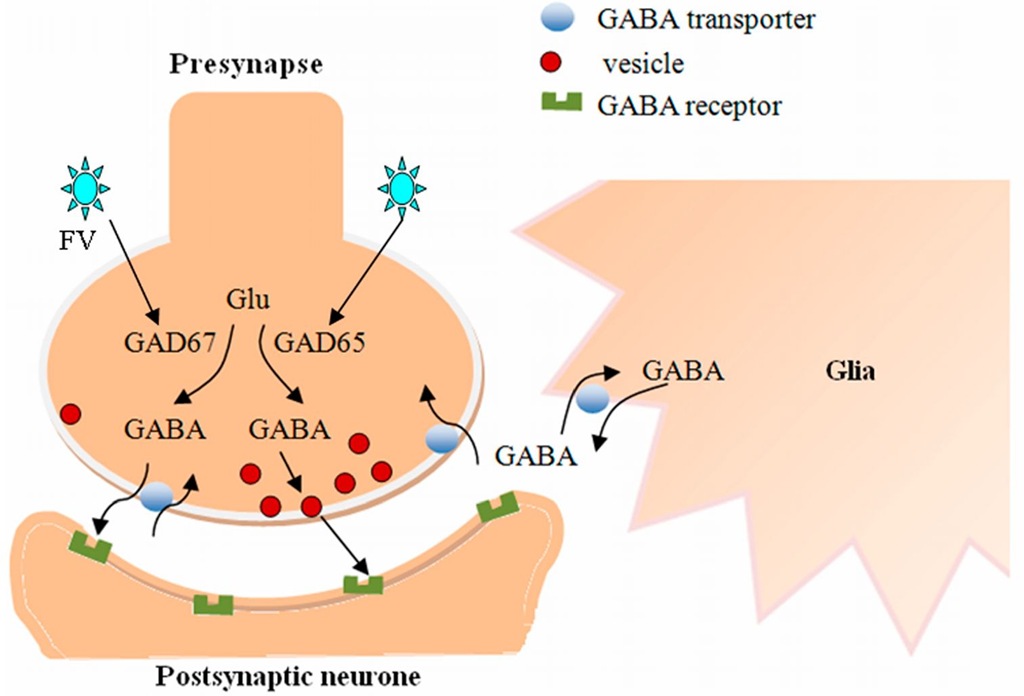
Parkinson’s disease (PD) is the second most common age-related progressive neurodegenerative disorder (Feng et al., 2010). Currently, the treatment of PD is focused on the amelioration of symptoms and does not have a satisfactory therapeutic effect. Caprariello and colleagues demonstrated that high-titer FV, which is able to efficiently transduce brain parenchyma, had the potential for gene therapy of disorders of the central nervous system (CNS) (Caprariello et al., 2009). FV-vector-mediated gene transfer to neural progenitor cells that are potential vehicles for delivery of therapeutic agents into the brain can achieve differentiation-dependent gene expression (Rothenaigner et al., 2009). In a rat model of PD, replication-defective HFV vectors were used to transduce astrocytes, which were then injected into the subthalamic nucleus (STN) region of PD animal models. The HFV vector-derived glutamic acid decarboxylase (GAD) expression in the astrocytes resulted in behavioral recovery of the rats. The transduction of the GAD vector achieved isoform-specific expression of GAD, synthesis of a significant amount of gamma-aminobutyric acid (GABA) and the release of tonically active GABA (Liu et al., 2007). GABA is primarily synthesized from glutamate (glutamic acid) by the pyridoxal-5-phosphate-dependent glutamic acid decarboxylase. GAD65 and GAD67 are two isoforms of GAD in the brain, which provide a dual system for the control of neuronal GABA (Figure 4). GAD65 is mainly present as an inactive apoenzyme and can be induced by nerve activity, whereas GAD67 is a pyridoxal phosphate-bound permanently active holoenzyme (Lindefors, 1993). In this study, replication-defective vector (rdv) GAD67 or rdvGAD65 were injected into the rat STN. There was a significant decrease in the rotation rates of the rdvGAD65 rats while the rdvGAD67 rats did not. This study demonstrates that HFV vector-derived GAD expression in astrocytes provides a potential approach to repair GABA transmission in neurological disorders (Liu et al., 2007).
Fig. 4. GABA is primarily synthesized from glutamate (glutamic acid) by GAD67 and GAD65. GAD67, which might preferentially synthesize cytoplasmic GABA, appears to be distributed more uniformly in neurons. GAD65, which might preferentially synthesize GABA for vesicular release, tends to be concentrated in nerve terminals. In most physiological circumstances, GABA-uptake transporters rapidly remove GABA that is released from the synapse into the extracellular space. However, the GABA transporter can reverse its action and release GABA from neurons or glial cells under certain conditions. FV vectors encoding GAD65 or GAD67 can achieve significant synthesis and release of GABA, which may ameliorate neurological disorders associated with hyperexcitable or diminished inhibitory activity.
Neuropathic pain
Neuropathic pain is an important health concern that is often refractory to medical management. Further studies of neuropathic pain have made the development of gene therapy a possibility. Subcutaneous inoculation of a replication-defective HFV vector expressing GAD67 attenuated below-injury level central neuropathic pain after spinal cord injury (SCI). To achieve the release of GABA, the GAD67 gene was transferred into dorsal root ganglion (DRG) cells for 7 days after T13 spinal cord hemisection. The result suggests that HFV-mediated gene transfer to DRG could be applied to treat below-injury level central neuropathic pain after incomplete SCI (Liu et al., 2008). The inhibitory effect of GABA, which is the major inhibitory neurotransmitter in the mammalian central nervous system, is mediated by GABAa, GABAb and GABAc/GABAa-p receptors (Rissman et al., 2011). GABA-mediated depolarization influences the excitability of sensory neurons both in cell bodies and nerve terminals by inactivating other voltage-sensitive channels, such as Ca2+ and Na+. It is also possible that the GABA-activated a Cl- current directly that inhibits an ATP-evoked excitatory current in DRG neurons (Naik et al., 2008).
Leukocyte adhesion deficiency
Gene transfer into hematopoietic stem cells (HSCs) is a promising treatment approach for many hematologic and genetic diseases. FV vectors can overcome safety concerns and the low HSC transduction rates found with oncoretroviral vectors. Recombinant FV vectors efficiently transduced human umbilical cord blood CD34+ cells that were injected into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice (Leurs et al., 2003; Josephson et al., 2002; Josephson et al., 2004; Zucali et al., 2002). The transduced human cells expressed high levels of the transgene in lymphoid, myeloid, and progenitor cells (Leurs et al., 2003; Vassilopoulos et al., 2001; Josephson et al., 2002). Additionally, there was no transgene silencing (Vassilopoulos et al., 2001; Josephson et al., 2002). Compared with FV, human immunodeficiency virus type 1 (HIV-1)-based lentiviral vectors pseudotyped with gibbon ape leukemia virus envelope (GALV Env) and murine leukemia virus (MLV)-based oncoretroviral vectors were inefficient in transducing NOD/SCID repopulating cells (Leurs et al., 2003). Bauer et al. demonstrated that FV vectors expressing the canine CD18 gene from an internal murine stem cell virus (MSCV) promoter could correct the lymphocyte proliferation and neutrophil adhesion defects characteristic of canine leukocyte adhesion deficiency (LAD). This work was the first successful use of FV vectors to treat a genetic immunodeficient disease. In addition, genotoxic complications were not observed, and compared with gammaretroviral vectors, integration site analysis revealed a polyclonality of transduced cells and a decreased risk of integration near oncogenes (Bauer et al., 2008). In another study, an FV vector expressing canine CD18 from a phosphoglycerate kinase (PGK) gene promoter, without an enhancer that would activate neighboring genes, expressed CD18 efficiently in canine neutrophils and CD34+ cells. However, dogs continued to suffer from LAD after treatment of hematopoietic stem cells transduced with the PGK-CD18 vector. This suggests that the PGK promoter cannot effectively replace the MSCV promoter in CD18-expressing FV vectors and that a strong promoter-enhancer may be necessary in FV vectors for the treatment of human LAD (Bauer et al., 2011).
Cancer
Selective introduction of a foreign gene into tumor cells to produce an enzyme is the basis of suicide gene therapy as an anticancer strategy. The gene product activates an inert prodrug to its cytotoxic form, which results in tumor cell death (Bhaumik, 2011). To evaluate the effect of tumor growth suppression utilizing suicide gene therapy, FV vectors expressing the purine nucleoside phosphorylase (FOV-7/ pnp), the nitroreductase (FOV-7/ntr), or the thymidine kinase (FOV-7/ tk) suicide genes were injected into the nude-mouse/human subcutaneous U87 glioblastoma xenograft model. Mice with vector virus-injected tumors were treated with the respective prodrug, resulting in a significant inhibition of tumor growth. Without prodrug treatment, a similar suppression of tumor growth was also observed both in mice with vector virus-injected U87 tumor cells and in the G59 glioma model that received the FOV-7/ pnp virus vector. Furthermore, wild-type FV, instead of the suicide gene-transducing vectors, wasable to inhibit tumor growth, suggesting an oncolytic activity of foamy virus replication in a nude-mouse glioblastoma xenograft tumor model. However, the vector is not restricted to the tumor and persists in various mouse tissues. This persistence limits its potential use in clinical trials (Heinkelein et al., 2005). Replication-defective HFV vectors expressing interleukin-24 also exhibited an inhibitory effect on cancer cells (Chen et al., 2010).
Acquired immunodeficiency syndrome
FV vectors have the potential for gene therapy of acquired immunodeficiency syndrome in patients who are resistant to traditional antiviral therapy. The nonpathogenic HFV mediated intracellular expression of ovine interferon-tau permitted cells to be resistant to HIV infection (Fujii et al., 2004). Vectors developed from SFV expressing anti-rev/ env (R2) short-interfering RNA (siRNA) effectively inhibited simian immunodeficiency virus (SIV) replication. This result shows that R2 siRNA reduces the rev and env gene expression and is a potent inhibitor of SIV replication (Park et al., 2005). Taylor and co-workers used three anti-HIV transgenes, including a dominant negative version of the viral rev protein (RevM10), a short hairpin RNA directed against a conserved overlapping sequence of the tat and rev genes (Sh1), and a membrane-attached peptide blocking HIV cell entry (maC46). These transgenes, expressed by foamy virus vectors both individually and collectively, were used to determine if they were able to effectively block HIV replication in macrophages. HIV replication was specifically blocked by maC46 or sh1 transgene expression. Entry inhibition by the maC46 transgene was the most effective method of blocking HIV replication among these three individual transgenes. In addition, the three anti-HIV transgenes expressed by FV vectors together effectively blocked HIV infection in primary macrophages derived from transduced, peripheral blood CD34-selected cells and in a cell line used for propagating HIV (Taylor et al., 2008).
Hepatitis B Virus (HBV)
Double-stranded RNA initiates and directs sequence-specific, post-transcriptional silencing of homologous genes. The use of RNA interference (RNAi), which is mediated by double-stranded small interfering RNA (siRNA), has received attention for the treatment of infectious diseases caused by viral or parasitic infection (Arenz et al., 2003). To successfully apply RNAi in the treatment of HBV infection, it was necessary to screen specific RNAi targeting sequences that could effectively knock down HBV transcripts. In a cell-based HBV infection model, two effective siRNA sequences designated S2 and X1 were cloned into HFV-based vectors to produce single siRNA expression vectors (HFVU6-siS2, HFVU6-siX1) and a dual siRNA expression vector (HFVU6-siSX). These siRNA vectors achieved long-term inhibition of HBV gene expression and viral DNA replication. HFVU6-siSX simultaneously expressing the two siRNAs that targeted the S and X genes of HBV was the most potent inhibitor of HBV replication, suggesting HFVU6-siS2 and HFVU6-siX1 may cooperate to inhibit HBV mRNA expression (Sun et al., 2007).
Chronic granulomatous disease
Chronic granulomatous disease (CGD) is a fatal genetic defect in leukocyte function caused by mutations in any of the four genes encoding the subunits (p22phox, gp91phox, p47phox and p67phox) of phagocyte NADPH oxidase (Kume et al., 2000; Chatziandreou et al., 2011). The majority of CGD cases are due to sex-linked recessive inheritance resulting from mutations in the CYBB gene encoding gp91phox. Chatziandreou et al. evaluated the gene transfer potential of FV vectors in an X-linked form of chronic granulomatous disease (X-CGD). FV vectors expressing the human codon-optimized gp91phox reconstituted NADPH activity in vitro in the X-CGD cell line, ex vivo in primary murine HSCs and in vivo in the X-CGD mouse model of the disease. Sustained long-term expression of the gp91phox transgene and a high percentage of superoxide-producing cells in the peripheral blood of transplanted X-CGD mice was achieved by FV vectors, suggesting that FV-based vectors were effective therapeutic vehicles for the genetic correction of X-CGD (Chatziandreou et al., 2011).
Perspectives
Because foamy virus infections are nonpathogenic, FVs have been considered potential vectors for the advancement of gene therapy. Moreover, the discovery of the internal promoter, IP, and the transactivator, Tas, have greatly promoted the research on foamy viruses. However, new issues have arisen in the course of foamy virus research; for example, studies have mainly focused on the primate foamy virus, which limits the field. Additionally, the products and functions of the structural and regulatory genes are not completely understood. Furthermore, although FVs are widespread, the host cell receptors are not yet known. These unknown features make the development of foamy virus gene therapy vectors difficult, yet there is still an interest in their development.
The use of viral mediated gene transfer is challenged by serious defects, yet they remain a potential tool of gene therapy for various diseases (Bastone et al., 2007). Therefore, it is critical to find a refined vector that is efficient and safe for treatment of the diseases, especially for nervous system therapy. To date, it is believed that FVs are nonpathogenic to the host, and their regulation of gene expression has been widely studied. Foamy virus is a widespread retrovirus with a unique structure that is innocuous in both human and primate infections. Thus, compared with the four major viral vector systems, the FV vectors appear to be a harmless and capable vehicle. With the increased understanding of neurological diseases and the improvement of gene therapy, the potential future of FV-based vectors carrying therapeutic genes for nervous systems and other organic systems is bright.