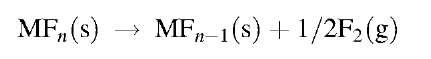INTRODUCTION
Since the soccer-ball-shaped molecule C60 and other fullerenes became available in macroscopic quantities, a wide variety of derivatives with multiple C-F bonds have been generated and observed in the gas phase. A significant number of these have also been isolated in weighable amounts and characterized by spectroscopic, electrochemical, and diffraction techniques. Those that have had their structures unambiguously determined by single-crystal X-ray diffraction include the fluoro-fullerenes C60F18, C60F36, and C60F48; the trifluoromethyl derivative C60F17(CF3); the oxafluorofullerene C60F18O; and the organofluorofullerene C60F15[CBr(CO2Et)2]3. In this article, the major developments in fluorofullerene chemistry will be reviewed, including the synthesis and characterization of selectively fluorinated fullerenes and their physical properties and chemical reactivities that will be important in their consideration for technological applications.
BACKGROUND
Fluorination was among the first derivitizations proposed for buckminsterfullerene, C60, the new allotrope of carbon discovered in laser-vaporized graphite in 1985.[1] Although it was only known as a gas-phase species at the time, the discoverers speculated that the preparation of fully fluorinated ”Teflon-like” ball bearings (e.g., C60F60) might lead to a new era of ”superlubricating” materials. Not surprisingly, when C60 became available in macroscopic quantities in 1991,[2] its reaction with F2 was immediately investigated.[3] Extensive experimental work followed, with dozens of publications from experts in the specialized and technically demanding field of fluorine chemistry.a
The history of fluorofullerene (FF) chemistry can be divided into two periods, the first being the initial exploration period from 1991 to 1994. Commonly available fluorinating reagents and well-known methodologies were employed during this time.a The substrate for these seminal papers was either a few milligrams of pure C60 or larger amounts of the so-called ”fullerene extract,” which was predominantly C60 with the bulk of the remainder being C70. The purported FF products proved difficult to characterize. Nevertheless, approximate numbers of F-atom substituents (formerly referred to as F-atom ad-dends[4]) were reported. Also reported were relative stabilities in various solvents, thermal stabilities, relative solubilities, and, to a limited extent, chemical reactivities, although these early FF products were not pure compounds of definite composition. They were complex, often intractable mixtures, in most cases containing a broad distribution of C60Fx and C70Fx molecules. Furthermore, it is now known that some early samples contained significant amounts of oxafluorofullerenes, C60FxOy (y =1, 2[5] or y = 1-18[6]), making the product mixtures even more complex than originally thought. For these reasons, the early literature should be examined with skepticism and cited with caution. The frequently cited Nature paper ”No Lubricants from Fluorinated C60” is a good example.[7] The rapid hydrolysis and low stability of FF samples was reported, but it was the abundant, much more reactive C60FxOy ”impurities,” and not the simple C60Fx FFs, that turned out to be thermally labile and to undergo rapid hydrolysis.[6] Another example is a current website that describes the putative C60F60 FF as a ”colorless,” ”crystalline solid” with a ”melting point of 287°C,”[8] although the original reports of its preparation and isolation are now widely believed to be incorrect.[9] As discussed below, the most highly fluorinated [60]fullerene that has been isolated is C60F48. The hypothetical molecule containing a closed C60 cage and having one F atom attached to each C atom has not been shown to exist, reports of its aforementioned physical properties (and pending commercial availability!) notwithstanding.
The second period, from 1994 to the present, has been a period of deliberate improvements and innovations in synthetic strategies that resulted in the isolation and complete characterization of several FFs that are 90+% compositionally pure, including one that is also isomeri-cally pure to within the limit of detection.b The breakthrough that inspired this work was the synthesis and 19F nuclear magnetic resonance (NMR) characterization of ~ 70% compositionally pure C60F48 from C60 and F2 (i.e., direct fluorination).[10] Its detailed structure (i.e., the positions of the 48 C-F bonds and the 6 remaining C-C double bonds) could not be unambiguously determined from the NMR data. Assuming, as the authors correctly did, that C60F48 was an intact C60 cage with 48 F atoms attached to 48 of the 60 C atoms, 14 different isomers were consistent with the NMR data (the total number of possible isomers for C60F48 is greater than 20,000,000). They suggested that the two most likely structures, one with D3 symmetry and one with S6 symmetry, were the two predicted by MO calculations to be the most stable thermodynamically, although it was not known whether the fluorination reaction was under thermodynamic or kinetic control. The importance of this paper, overlooking the low yield and poor compositional purity, is that two important features of FF chemistry that served to inspire others had been determined: 1) the final product of direct fluorination is C60F48, so previously reported products with fewer than 48 F atoms represented incomplete fluoridations; and 2) fluorinated products of certain stable compositions can, in principle, be obtained. Further study of C60 direct fluorination led to the high-yield preparation of macroscopic amounts of compositionally pure C60F48,[11] which made possible its complete characterization by a battery of physicochemical techniques including X-ray crystallography[12] and combustion calo-rimetry, the latter providing the first experimentally determined enthalpy of formation for any fullerene derivative.
The next important development in the preparation of selectively fluorinated fullerenes was reported in 1995 by chemists at the Moscow State University. They used transition-metal fluorides as fluorinating reagents and observed the formation of the selectively fluorinated fullerenes C60F18 and C60F36.[14] This was followed by papers describing the laborious chromatographic separation of dozens of less-abundant products, including FFs with x=2, 4, 6, 8, 16, and 20,[15-21] as well as oxafluoro-fullerenes[22-24] and fullerenes containing both F atoms and CF3 groups.[23,25] For the first time, detailed studies of the physical and chemical properties of gram quantities of individual, compositionally pure FF compounds could be carried out, studies that must precede the development of commercial applications of any new compound.
SYNTHESIS OF FLUOROFULLERENES
From C60 and F2, XeF2, KrF2, ClF3, or BrF5
Until 1995, direct fluorination with F2 was the most extensively studied method of C60 fluorination.[26] Many different experimental conditions were investigated: reaction temperatures from 25°C to 355°C; a 1-atm continuous-flow reactor or a high-pressure batch reactor; and reaction times from hours to months. In some cases, the in situ mass uptake of the sample was recorded[3] or the volatile products were monitored by mass spectrometry1-27-1 or infrared (IR) spectroscopy.1-3-1 In others, color changes from black to brown to pale yellow to white were noted.[28,29]
Direct fluoridation occurred slowly at 25°C (weeks), affording mixtures of unreacted C60 and C60Fx FFs with even x values from 36 to 46.[30] Similar results, but with higher overall conversions, were obtained at 130-250°C.[29,31] A complicated two-stage direct fluoridation at 250-275°C in the presence of NaF afforded a 56% yield of FF material in 50 hr that was 70 mol% C60F48 according to mass spectrometry.1-10-1 In contrast, a one-stage direct fluoridation in a flow reactor at 315-355°C afforded a 70% yield of FFs in 3-10 hr that was 95 mol% C60F48 according to electron-ionization mass spectrometry (EI-MS).[31- This is still the highest compositional purity reported to date for C60F48.
Use of the noble gas fluorides XeF2 and KrF2, which are more powerful fluorinating reagents than F2, did not afford compositionally pure C60F48. In the case of XeF2, complex mixtures of FFs were isolated.[28,32- In the case of KrF2, a mass spectrum of the complex mixture of fluorocarbon products showed the presence of nonfuller-ene C60Fx species with x>60, which requires C60 cage rupture and the formation of CF2 and/or CF3 groups.[33] Such hyperfluorinated species had been previously observed as products of the direct fluorination of C60 under UV radiation.[34]
The use of the reactive interhalogens ClF3 and BrF5 did not result in significant yields of any compositionally pure FF.[5] To further complicate matters, many products containing multiple Cl or Br atoms in addition to F atoms were obtained based on IR spectroscopy and elemental analysis. It was also reported that ”IF7 did not react [with C60] in the gaseous phase.”[5]
The reaction of C60 dissolved in liquid Br2 (which is known to produce C60Br24) with BrF3 was reported to yield C60F24.[35] The stoichiometry was based on the ratio of C 1s and F 1s XPS (X-ray photoelectron spectroscopy) intensities. However, new, unpublished mass spectromet-ric evidence indicates that the product actually consisted of a distribution of C60Fx compositions with an average x value of 24 (N.F. Yudanov and O.V. Boltalina, unpublished data, 2001).
The reaction of C60 with IF5 in liquid CCl4 was reported to yield chlorofluorofullerenes, including C60Cli8-F14.[36] However, the only method of characterization was low-resolution mass spectrometry, and these data were inconclusive. For example, a sample of C60Cl24 and a sample purported to be C60Cl18F14 exhibited similar complex distributions of ions with m/z at 1534±2 Da, 1464±2 Da, 1394 Da, 1322±2 Da, etc.
From C6o and High-Valent Metal Fluorides
A conceptual breakthrough in the synthesis of selectively fluorinated fullerenes was discovered in 1995, not by synthetic chemists but by mass spectrometrists at Moscow State University (MSU) who were studying the thermochemistry of gaseous ions.[14] They used a magnetic-sector mass spectrometer equipped with a Knudsen effusion cell as a chemical reactor. Solid state reactions of fullerenes with various high-valent transition-metal and rare-earth-metal fluorides as fluorinating reagents resulted in the simultaneous generation and mass-spectrometric detection of the volatile products. Because the temperature was programmed to increase over time, a wealth of information on the dynamics of formation and distribution of combinatorial-like mixture of products as a function of temperature was obtained for each pair of reagents and stoichiometric ratio studied. Significantly, a large fraction of the products formed at a given temperature were condensed on a specially designed collection plate, which allowed for the further characterization of new derivatives by a variety of spectroscopic techniques.[15,16]
The Knudsen-cell technique was largely responsible for the rapid development of synthetic fluorofullerene chemistry. It was discovered that heating a 1:36 mole ratio mixture C60 and MnF3 afforded a 60-70% yield of 90+ % compositionally pure C60F36.[37] Another paper reported that mixtures of C60 and the ternary metal fluoride K2PtF6 resulted in the selective formation of C60F18 (also with 90+% compositional purity and in 60-70% yield) with only trace quantities of C60F36 and several other FFs with fewer than 18 F atoms present.[38] In general, reactions with high-valent metal fluorides gave products with high compositional purity or, at worst, a narrow distribution of C60Fx compositions. In general, it was also found that the average number of F atoms added to C60 is correlated with the relative fluorinating strength of the metal fluoride, which to a first approximation increases as the enthalpy change for the reaction
becomes less positive or more negative.[39] Additionally, it was found that, for a given metal fluoride, higher temperatures favored the selective formation of FFs with fewer F atoms per C60 cage. Some representative data are listed in Table 1.
A recent paper reported a series of reactions of C60 with ternary metal fluorides other than K2PtF6.[40] This is potentially a significant next step for two reasons. First, ternary metal fluorides are more numerous than the corresponding binary transition metal fluorides (consider K2MnF6 and Rb2MnF6 vs. MnF4 and CsPbF6 and CsPbF6 vs. PbF4), so a wider variety of reagents has become available to the synthetic chemist. Second, these ternary compounds are generally weaker fluorinating reagents (i.e., less reactive) than the parent binary metal fluoride. This feature makes them easier to handle in the laboratory environment and, more importantly, opens up the possibility of isolating FFs with lower degrees of fluorination.
Table 1 Values of x for the CyFx or C59NFx major products of fullerene fluorinationsa
|
Fullerene substrate |
|
|
Fluorination reagent (temperature range, °C) |
|
|
|
F2 (25-300) |
AgF2 (330-500) |
MnF3 (380-500) |
CeF4 (420-520) |
K^PtFg (450-520) |
|
|
C60 |
48 |
44 |
36 |
36 |
36, 18 |
|
C70 |
56/54 |
40/38/36 |
|||
|
C74 |
48/46 |
40/38/36 |
38, 18 |
||
|
C76 |
54/52 |
40/38 |
42/40/38 |
38/36, 20 |
|
|
C84 |
58/56 |
44/42 |
42/40 |
40/38, 24 |
|
|
C59Nb |
37/35/33 |
35/33 |
33/31, 17 |
||
|
Percentage of occupancy, |
67-80 |
62-73 |
49-60 |
48-60 |
24-30 |
|
(x/y) x 100%c |
The reaction of C60 with WF6, TaF5, NbF5, and TiF4, which are not particularly strong fluorinating reagents, only produced ”adducts” of unknown structure [e.g., C60(MF„)x].[41] No fluorofullerenes were observed.
Fluorination of Higher Fullerenes and Some C6o Derivatives
Fluorination of the higher fullerenes C74, C76, C78, and C84 and of C60 derivatives such as (C59N)2, C60Clx, and C60Brx were investigated as these compounds became available in macroscopic quantitites. As far as the higher fullerenes and (C59N)2 are concerned, EI-MS characterization of volatile products generated by the Knudsen-cell technique showed that specific compositions, or narrow ranges of compositions, could be attained with a given high-valent metal fluoride and a given reaction temperature.1-42-44 Representative data are listed in Table 1. Note that the percentage of occupancy for the CyFx and C59NFx compounds with the smaller of the listed x values parallels the percentage of occupancy for the C60Fx products with the same fluorination reagent. Some individual higher FFs and C59NFx compounds have been isolated, but none has been extensively characterized. Nevertheless, the fact that different x values or narrow ranges of x values were observed in each case means that certain CyFx and C59NFx compounds may possess a special kinetic or thermodynamic stability similar to the special stabilities possessed by C60F18, C60F36, and C60F48. Based on this reasoning, likely structures of C74F38 and C76F20[44] and several C59NFx compounds[45] have been proposed.
Reactions of the halofullerenes C60Br6, C60Br8, C60Br24, C60Cl6, and C60Cl24 with either F2 or XeF2 were reported to produce a variety of FFs, mixed fluorohalo-fullerenes, and/or oxafluorofullerenes.[36] Another study of the reactions of C60Br8 and C60Br24 with XeF2 reported similar findings.[32] An important goal of these studies was to prepare compositionally pure FFs such as C60F6, C60F8, and C60F24 that have not yet been prepared by other methods. However, to date, there is no convincing example of a direct, high-yield C60Xx! C60Fx transformation (X=Cl, Br).
STRUCTURAL CHARACTERIZATION OF FLUOROFULLERENES
X-Ray Crystallography
The fluorofullerenes and fluorofullerene derivatives that have been structurally characterized by single-crystal X-ray diffraction are all derivatives of C60. The complete list includes the three FFs, C60F18, C60F36, and C60F48, and the three FF derivatives, C60F17(CF3), C60F18O, and C60F15[CBr(CO2Et)2]3. From these few structures, an analysis of F-atom addition patterns and of interatomic distances and angles has provided some insight into the mechanisms by which F atoms and other atoms or groups of atoms are added to a given starting material or intermediate. An assessment of the distances and angles also provides insight into the steric and electronic reasons that the underlying C60 cage is distorted, in some cases significantly distorted, from the nearly spherical Ih symmetry of native C60.
C60F18
This was the first FF to be structurally characterized.[46] Seven different modifications have been studied, a solvent-free modification,1-38-1 a toluene solvate,[46] and five other arene solvates.[47] They all contain the same C3v isomer of C60F18. A ball-and-stick plot (BSPlot) of the C60F18 molecule present in the toluene solvate, and its Schegel diagram, are shown in Fig. 1. The molecule has all 18 F atoms situated on one hemisphere. This undoubtedly makes it highly polar (calculated dipole moments range from 12 to 16 D.)[46] The Schlegel diagram shows that the 18 F atoms have been added to a contiguous set of C atoms, a common feature of hydro-fullerenes but uncommon when larger substituents are present, as in chlorofullerenes and bromofullerenes.[48] The Schlegel diagram also shows that the fluorinated hemisphere contains a central, nearly planar, fully aromatic benzenoid ring, with six nearly identical C-C distances, separated from the remainder of the fullerene p-electron system by a belt of sp3 C atoms each bearing a C-F bond. The BSPlot shows that the fluorinated hemisphere is significantly flattened, so much so that if the unfluorinated hemisphere is superimposed on a molecule of C60, the C atoms of the benzenoid ring are ca. 0.6 A closer to the center of the C60 molecule than the C atoms of the antipodal hexagonal ring. The C-F distances are normal. The C(sp3)-C(sp3) distances, many of which are greater than 1.62 A, are significantly longer than typical organic C-C distances but are not unusual for highly fluorinated or perfluorinated hydro-carbons.[46]
Fig. 1 Ball-and-stick plots and Schlegel diagrams for three structures determined by X-ray crystallography. The open and hatched circles in the plots represent C and F atoms, respectively. The solid circle in the plot of C60F18O represent the O atom. The solid circles in the Schlegel diagrams represent C-F bonds.
c6c>Fi7(CF3)
This compound was a by-product of C60 fluoridation with K2PtF6. It was originally believed to be Cg0F18(CF2), with the CF2 group incorporated into a cyclopropane ring.[49] The true nature of the structure was revealed by X-ray diffraction analysis. Crystals of this compound were found, both by X-ray and 19F NMR analysis, to contain a Cs isomer (ca. 68%), the Schlegel diagram of which is shown in Fig. 1, and a pair of C1 enantiomers (ca. 16% each) having the CF3 group attached instead to one of the six symmetry-related sp3 C atoms that are not directly bonded to the central benzenoid ring.[50] All of the isomers exhibit threefold rotational disorder in the solid state. Nevertheless, a reasonable structure solution was possible. A BSPlot of the major isomer is shown in Fig. 1. The molecule exhibits the same flattening observed in the structure of C60F18. It may be mechanistically significant that the major isomer of C18F17(CF3) has the CF3 group attached to the least sterically demanding sp3 C atoms.
c6cf18o
As was the case with C60F17(CF3), the X-ray diffraction analysis of the oxafluorofullerene C60F18O settled a longstanding problem concerning its structure. It was also a by-product of C60 fluorination with K2PtF6. It had been originally formulated as an epoxide,[6] an interpretation that remained unchallenged nearly a decade later.[20,51-53] The structure, shown in Fig. 1, revealed that it is an ether formed (conceptually, not necessarily mechanistically) by the insertion of an O atom into a C(sp3)-C(sp3) bond of C60Fi8.[22] The structure shown is the major isomer of C60F18O; two other isomers were separated by high-performance liquid chromatography (HPLC) and are presumably also ethers, with the O atom inserted into different C(sp3)-C(sp3) bonds. Note that in the major isomer, the O atom has been inserted into one of the three longest C(sp3)-C(sp3) bonds, which average 1.67 A in C60F18. The two remaining such bonds in C60F18O are probably also extremely long, but the threefold rotational disorder in these crystals makes distances and angles involving these C atoms unreliable.
C6oFis[CBr(CO2Et)2]3
This compound, originally prepared and characterized by mass spectrometry (A.L. Mirakyan, personal communication to O.V. Boltalina, 2000), was later purified and structurally characterized by single-crystal X-ray dif-fraction.[54] Its structure is related to that of C6qFi8 except that the three bulky organic substituents are not attached to C atoms contiguous with the belt of 15 sp3 C atoms.
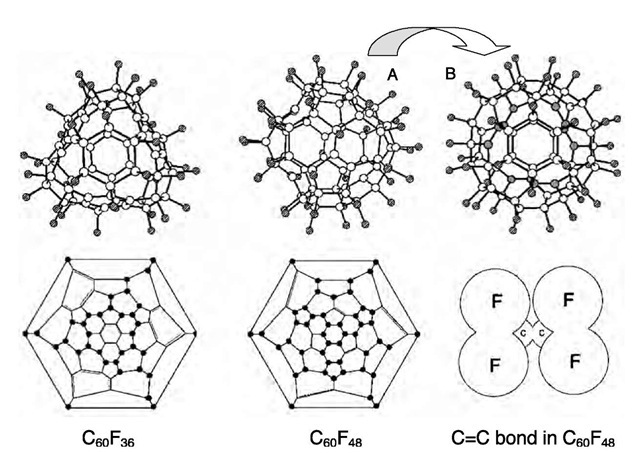
c60 f36
There are three isomers known for this composition. The two major isomers have C3 (>90%) and T (<10%) symmetry.[55] A BSPlot and Schlegel diagram of the T isomer are shown in Fig. 2.[56- This structure is severely disordered, so much so that all 36 C-F bonds had to be constrained to have the same distance. There were other constraints imposed on the two independent C60F36 molecules in the asymmetric unit. Nevertheless, four salient features were unambiguously determined. First, this isomer of C60F36 does indeed have idealized (although not crystallographic) T symmetry, in agreement with predictions based on 19F NMR spectra.[55,57] In accordance with its proper rotational symmetry, T-C60F36 is chiral and exists as a pair of enantiomers. Second, the 36 F atoms were added to a contiguous set of C atoms, which is consistent with the addition patterns extant in C60F18, C60F17(CF3), and C60F18O. Third, there are four isolated benzenoid rings in this isomer, each of which is planar to within ±0.02 A. The Schlegel diagram in Fig. 2 shows that any three of the four benzenoid rings are symmetrically related by a C3 axis perpendicular to the plane of the remaining ring. Fourth, the molecule is severely distorted from the nearly spherical structure of C60. The four benzenoid rings are much closer to the geometric center of the molecule than the corresponding six-membered rings in C60, while the C6F6 hexagons opposite the benzenoid rings are much farther away from the geometric center. In C60, the distance from each C atom to the geometric center is 3.57 A; in T-C60F36, the corresponding distances from the geometric center to the benzenoid C atoms and to the antipodal C6F6 C atoms are 3.0-3.1 and 3.9-4.0 A, respectively.
The structure of the C3 isomer has not yet been determined by X-ray diffraction. Its likely structure, deduced from a careful analysis of 1-D and 2-D 19F NMR spectra, has three benzenoid rings and three isolated C=C double bonds arising from a contiguous addition of F atoms.[57] If one of the double bonds were moved by just one C atom, the C3 axis would be lost and the molecule would have C1 symmetry. This isomer has recently been isolated and structurally characterized by X-ray diffraction.[58]
Fig. 2 Ball-and-stick plots and Schlegel diagrams for the T-symmetry isomer of C60F36 and the D3-symmetry structure of C60F48, both determined by X-ray crystallography. Two different rotational orientations, A and B, are shown for C60F48. In orientation B, looking down the C3 axis, the 12 remaining sp2 C atoms are represented as grey circles and form the six isolated C=C double bonds, three in the upper hemisphere and three in the lower hemisphere. Elsewhere, the open and hatched circles represent C and F atoms, respectively. The solid circles in the Schlegel diagrams represent C-F bonds. Also shown is the local structure around one of the C=C bonds in D3-C60F48. The size of each F atom in this drawing corresponds to its van der Waals radius.
c60f48
Single crystals of this compound, grown from mesitylene solution, were found to contain nearly equal amounts of D3 and S6 isomers. The X-ray diffraction analysis was further complicated by the fact that both isomers appeared to be statistically disordered over two positions. The structure was eventually solved, and a BSPlot and Schlegel diagram of the D3 isomer are shown in Fig. 2.[12- As in the other FFs and FF derivatives that have been structurally characterized, the 48 F atoms have been added to C60 in a contiguous manner. There are only 12 remaining sp2 C atoms distributed over six isolated C-C bonds, each of which is a common edge to a hexagon and a pentagon, the same as for the three isolated C-C bonds in the C1 and C3 isomers of C60F36. Similar to the structure of T-C60F36, the structure of C60F48 is severely distorted. The distances between the 12 sp2 C atoms and the geometric center are all ca. 3.05 A, while the distances between sp3 C atoms that are bonded only to other sp3 C atoms are all ca. 3.9 A.
Perhaps the most interesting feature of the structure is that the six remaining C-C bonds are well shielded from external reagents, both by the distortion that draws them closer to the molecular center and by their four adjacent C-F bonds. A diagram showing one of the isolated C=C bonds in C60F48 and its four closest F atoms is also shown in Fig. 2. The size of each F atom corresponds to its van der Waals radius. The four F atoms are nearly coplanar and form an idealized rectangle having two sides of 2.6 A, two sides of 3.3±0.2 A, and two diagonals of 4.1 A. It is rather obvious that the addition of more than 48 F atoms to an intact C60 cage must have a high kinetic barrier if not a high thermodynamic barrier.
Neither the D3 nor the S6 isomer has a permanent dipole moment. Therefore C60F48 may be the closest approximation to a spherical ”Teflon-like” ball prepared to date. It will be interesting to see what lubricating properties, if any, it possesses.