INTRODUCTION
Zeolites are three-dimensional, microporous, crystalline solids with well-defined structures that contain aluminum, silicon, and oxygen in their regular framework. When zeolite crystals are intergrown to form continuous layers, the resulting membranes can separate gas mixtures with high selectivities because their pore sizes are comparable to the molecular dimensions. Separation is possible by molecular sieving, preferential adsorption, or differences in diffusion rates. Zeolitic membranes can be classified as either symmetric membranes (self-supported) or asymmetric membranes (supported). Different from the meso-porous inorganic membranes used in gas diffusion process for uranium isotope separation, zeolite membranes consist of many crystallites packed together without crystallite boundary gap in ideal cases. Different types of membranes have varying crystal structure, pore size, and surface properties, thus exhibiting different separation and/or catalytic properties.
Up to date, some zeolite membranes such as the MFI type have been well developed in the last decade since some early works in this area.[1,2] Preparation techniques have been developed and fine-tuned in recent years, which makes it possible to fabricate a large and pinhole-free zeolite membrane. Zeolite-membrane-based separations1-3-1 and reactions1-4-1 have generated much interest in recent years. They are attractive for a variety of reasons, including steady state operation, tailored selectivity, low energy consumption, compatibility to high-temperature and high-pressure conditions, and potential for effective separation and combined reaction-separation systems. Such merits offer the opportunity to solve problems associated with high production costs. Zeolite membranes have been the highlight in the separation technology and novel reactor engineering fields, and is widely expected to find commercial applications in the near future. Some excellent reviews on the preparation and application of zeolite membranes have appeared in the last several years.[5,6] However, the rapid development of this field necessitates a timely update on this topic, particularly on preparation of zeolite membrane and its application to environmental separation and reactions.
TYPES OF ZEOLITE MEMBRANES
Zeolite, with its well-defined, nanometer-sized pore structure, is an attractive material for inorganic membrane separation and reactions. Small (i.e., 0.3-0.4 nm with 6-and 8-membered ring), medium (i.e., 0.5-0.6 nm with 10-membered ring), and large pore (i.e., 0.7-0.8 nm with 12-membered ring) zeolites have been successfully made into membranes.
SAPO-34 and NaA, having pore size windows of 0.38 and 0.43 nm, respectively, are examples of small-pore zeolite membranes. SAPO-34 zeolite membranes were reported by Poshusta et al.[7] to separate CO2/CH4, CO2/ N2, N2/CH4, H2/CH4, H2/CO2, and H2/N2 binary mixtures. NaA membrane has been intensively studied for dehydration of solvents and alcohols.[8] Their use in hybrid membrane reactor has been demonstrated for esterification reaction.[9] Recently, Xu et al.[10] and Dong et al.[11] used a novel process to synthesize a high-quality NaA membrane from clear solution. Besides, Xu and other researchers[12,13- investigated the effects of microwave heating on the synthesis of NaA zeolite membrane. Their results indicated that the microwave synthesis has the advantages of very short time, small zeolite particle size and narrow particle size distribution, and large permeance. Shah et al.[14] applied the NaA zeolite membrane to separate methanol/water, ethanol/water, and dimethylformamide/water mixtures with selectivities of 140, 2140, and 330, respectively.
The MFI structure (i.e., silicalite or its aluminosilicate analog, ZSM-5) has been most intensively investigated experimentally and theoretically and often employed in laboratory membrane devices. Owing to its medium-size 0.55 nm) pore network, which are close to the dimensions of industrial gases, it has been applied in many areas such as hydrocarbons separation, recycling of valuable gases[15] and novel metal complexes,[16] natural gas cleaning,1-17-1 membrane reactor, and so on. With their three-dimensional pore channel network, MFI zeolites are fairly robust and have been a main subject of research in zeolite membrane. Separations of light gases, close-boiling hydrocarbons, organic-water solution, and butane or xyl-ene isomers have been reported in the literature using MFI zeolite membranes. MFI zeolites have an anisotropic pore structure consisting of straight channels along the 010 crystallographic orientation intersected by sinusoidal channels along the 100 direction. This structural anisot-ropy is expected to influence the transport properties along the different zeolite crystallographic orientations. Foreign atoms can be easily substituted for Si in the MFI zeolite framework.[18] These include aluminum for ZSM-5, titanium for TS-1, and vanadium for VS-1.[19] This renders MFI zeolites a wider range of chemical and catalytic properties as compared to other zeolite families. Silicon-rich silicalite membrane is inert and hydrophobic, whereas the addition of Al in ZSM-5 transforms the zeolite into acidic catalyst with hydrophilic property. Similarly, the incorporation of titanium and vanadium in TS-1 and VS-1 impart the zeolites with unique catalytic properties. Besides modifying the zeolite chemistry, the presence of these foreign atoms in the framework also perturbs the size and shape of the zeolite pores affecting the transport of molecules through the channel. It also alters the chemical interaction between the diffusing molecule and the zeolite pore wall. The unsaturated charges caused by the insertion of foreign atoms are balanced by counterions, whose presence can further physically restrict and chemically modify the pore channels. Ge,[20] B,[21] V,[22] and Fe[23] atoms have been incorporated into the framework of MFI zeolite membrane with interesting properties exhibited.
Faujasite zeolites including zeolite NaY and NaX are representatives of large-pore zeolite (0.74 nm) membranes. Hence it can be used in applications involving larger molecules. Depending on their Si/Al ratios, fauja-sites are categorized into X (Si/Al«1-1.5) and Y (Si/ Al>2) although they both have topologically the same framework. Furthermore, modification of the faujasite crystals, either by ion exchange or by dealumination, can be used to control the adsorption or intracrystalline diffusion properties, providing a way of tailoring the membranes to specific applications. Hasegawa et al.[24] prepared ion-exchanged faujasite membranes that can separate CO2/N2 with a separation factor of 40. They also synthesized an NaY-type zeolite membrane on the inner surface of a porous a-alumina support tube by a hydrothermal technique. The synthesized Y-type zeolite membranes were stable at temperatures up to 400°C. Kita et al.[25] employed an NaY pervaporation membrane for the separation of methanol and methyl-tert-butyl ether (MTBE) mixtures. Nikolakis et al.[26] applied faujasite zeolite membrane to successfully separate benzene/cyclo-hexane, benzene/n-hexane, toluene/n-heptane, propylene/ propane, and ethylene/methane with the separation factors of 160, 144, 45, 6.2, and 8.4, respectively. Lassinantti et al.[27] described the preparation of FAU zeolite membrane on polished, seeded alumina wafers. The film thickness was found to be a linear function of synthesis duration up to a certain point. Further treatment reduced the film thickness. Continuous and crack-free films with thicknesses in the range 210-2670 nm were synthesized in their work. Jeong et al.[28] and Kobayashi et al.[29] studied the application of the NaY-type zeolite membrane to separate C6 hydrocarbon mixtures both experimentally and theoretically. Another large-pore zeolite, mordenite,[30-32] has also been used for H2/N2 and water/alcohol separation. Mordenite’s unidimensional, straight pore channels provide easy access and transport through the membrane, but make it more susceptible to fouling.
PREPARATION OF ZEOLITE MEMBRANES
In general, there are two categories of zeolite membranes mainly related to their preparation processes, i.e., self-supported membranes and composite membranes. The first type of membrane is constituted by a pure zeolitic phase, while a zeolitic thin film formed on a support is the composite type. The preparation of self-supported zeolitic films easily introduces some drawbacks in terms of lack of homogeneous thickness and mechanical instability. So far, zeolitic films have been synthesized in the presence of momentary support (therefore removed after the preparation) or permanent support (to form zeolite composite membranes). A momentary support can be a poly(tetra-fluoroethylene) slab, a Teflon sleeve, a Vycor frit, a silver, nickel, or stainless-steel plate. A permanent support can be any of the numerous available materials such as amorphous silica, silicon wafers, glass, aluminum platelets, stainless steel, porous ceramics, or porous a-Al2O3, g-Al2O3 membranes.
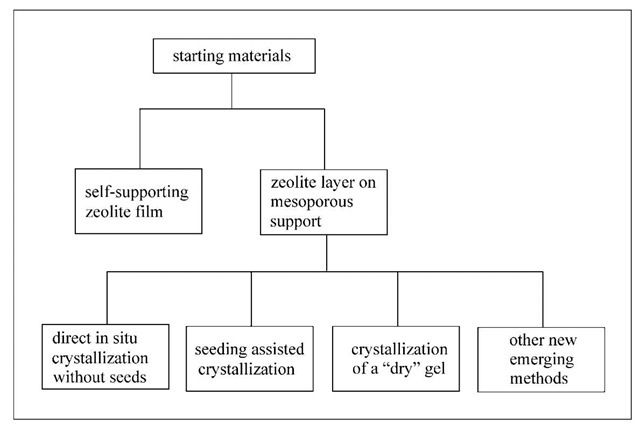
Freestanding zeolite layers larger than a few square centimeters are difficult to form, and the resulting structures are fragile. Therefore zeolite membranes are typi-callydeposited on mechanically robust porous supports (alumina, stainless steel, etc.). The technical challenge developing supported zeolitic membranes lies in how to decrease the large thickness (typically 50 mm) of the membrane required to obtain defect-free samples, because defects are easily presented in very thin layers. Several methods have been reported to grow zeolite films on porous supports by the in situ approach. Among the different strategies for the preparation of zeolite membranes, as shown in Fig. 1, the preparation of the so-called composite membranes seems to be the most frequently used and most promising technique.
Fig. 1 Preparation strategy for zeolite membrane.
The thin zeolite top layer can be hydrothermally crystallized in one step on the top of the support or inside the pores of the support, which can be called ”direct in situ crystallization.” The essence of the in situ approach is to bring the surface of a porous support in contact with a zeolite synthesis solution (sol or gel) and keep the system under controlled conditions so zeolite can nucleate and grow to a continuous film on the support surface. If the zeolite layer is formed in several steps using seed crystals that grow together to a continuous supported zeolite layer in a subsequent hydrothermal synthesis, the process is referred to as ”seeding supported crystallization.” In addition to the hydrothermal crystallization as the main technique to prepare supported zeolite layers, defect-free zeolite layers can be synthesized by a vapor-phase method called ”dry synthesis.” An amorphous dry gel can be crystallized on a porous alumina support by a vapor-phase transport method. Other new preparation concepts, such as pulsed-laser deposition,[33,34] microwave assisting synthesis,[12,13] and substrate heating method,[35] have been proposed in recent years.
ZEOLITIC MEMBRANES USED IN ENVIRONMENTAL SEPARATION
Zeolites have been conventionally used as catalysts, ion exchangers, and adsorbents. Molecules with different sizes and shapes can be discriminated or separated by zeolites through their channels. Generally, separation or purification of gases by zeolites is carried out by pressure swing adsorption. High capital costs and energy consumption characterizes the limitations of such process, and continuous operation is not easily realized. In a membrane configuration, both equipment and operation costs can be dramatically reduced, and continuous operation is possible. Since the 1990s, much research effort has been devoted to the synthesis of zeolite membrane. The potential of using zeolitic materials for membrane-based separations has been realized only in recent years. Uses of zeolite membranes in environment-friendly separations such as the production of clean fuel and energy-efficient processes are emerging. Among them, H2 separation from light gas mixtures and alcohol from aqueous solutions have received increasing attention. Some researchers have also studied other environment-relating separations. For example, Turlan et al.[16] applied silicalite membrane to recover a palladium homogeneous catalyst from its mixture with solvent and product. Piera et al.[36- used MFI-type zeolite membranes to separate CO/air at very low CO concentrations (160 ppbv). At 245 K, a CO/air separation factor of 3.14 was achieved, which shows that it is possible to use zeolite membranes for the removal of pollutants from air, especially where these gases are present in trace concentrations. This is the first work in the open literature dealing with gas separations at trace concentration levels using zeolite membranes.
H2 Separation from Gas Mixtures
Hydrogen as a clean energy carrier has attracted increasing attention worldwide because of their higher energy content, environmental advantages, and potential market. The greatest potential use for hydrogen is as an energy carrier for fuel cell vehicles. Moreover, the use of hydrogen presents a totally clean energy use without any emissions of NOx, CO, and CO2. However, H2 usually coexists with other light gases when it is produced from industrial processes such as gasification reactions and steam reforming reactions. Therefore it is highly desirable to separate hydrogen from other gases from hydrogen-rich gas streams. Membrane separation has been demonstrated to be superior than the present PSA technology. Studies in separation of H2 from light gases have been performed by many groups in recent years. Some key findings related to hydrogen separation are summarized in Table 1.
Smaller-pore zeolites such as zeolite A (0.38-nm pore diameter) and SAPO-34 (about 0.43-nm pore diameter) have the potential to improve light gas separation by exploiting differences in size between light gases. Several research groups[7,37,42- have explored the synthesis of small-pore zeolite membranes for the separation of light gas mixtures. They reported that H2/CH4 mixture was separated primarily by differences in molecular size, and the selectivity was about 4-25. Aoki et al.[38] reported that H2/N2 selectivities through NaA zeolite membranes were 3.7. Masuda et al.[39- develop a silane-cracking method to modify the pore size of MFI-type membrane, which was applied to H2 separation from mixture gases containing H2 and N2 or O2 in a flow system. The permeances of N2 and O2 of the modified membrane were markedly reduced to about 1/500 of those of a fresh membrane, whereas the permeance of H2 decreased to only 1/10 of that of a fresh membrane. Consequently, the separation factor of H2, which is 1.54.5 for a fresh membrane, could be enhanced to about 100. Illgen et al.[40- fabricated H2-selective MFI zeolite membranes on a porous ceramic tube. At 500°C, this membrane separate H2 from isobutane with separation factors of 70 and H2 permeances of ca. 1 m3 (STP)/m2 hbar. The high temperature is applied to eliminate the adsorption effects on the permeation. Lai and Gavalas[41] prepared the supported ZSM-5 membranes using a TPA-free synthesis gel on asymmetric a-Al2O3 tubular supports. The membranes have Si/Al ratio much greater than the membranes prepared using TPA+ in the synthesis gel. Gas permeation measurements yielded high selectivities based on the molecular size. At room temperature, the selectivities of H2 over N2, CH4, and n-butane were 38, 61, and 104, respectively.
Membranes for Energy-Efficient Alcohol/Water Separations
Alcohol/water separations are conventionally achieved by distillation, which is apparently inferior to membrane separation both in operating costs and energy consumption.
Table 1 H2 separation data from gas mixtures
|
Gases |
Membrane type |
Permeance [10 8 mol/(m2 s Pa)] |
Selectivity |
Reference |
|
H2/CH4 |
SAPO-34 |
5.0-17/0.48-3.8 |
4.5-13.5 |
[7] |
|
h2/ch4 |
SAPO-34 |
3.2/0.13 |
24.6 |
[37] |
|
H2/N2 |
A type |
5.0/1.3 |
3.7 |
[38] |
|
H2/N2 |
Modified MFI |
~ 2.5/ ~ 0.025 |
90-140 |
[39] |
|
H2/O2 |
Modified MFI |
- |
110-120 |
[39] |
|
H2/i-C4H10 |
MFI |
12.4/- |
70 |
[40] |
|
H2/N2 |
MFI |
2.6/0.068 |
38 |
[41] |
|
H2/CH4 |
MFI |
3.3/0.054 |
61 |
[41] |
|
H2/n-C4H10 |
MFI |
- |
104 |
[41] |
Table 2 Data for alcohol/water separations
|
Gases |
Membrane type |
Total flux of the permeate (kg/m2 ■ h) |
Selectivity |
Reference |
|
Ethanol/water |
A type |
1.10-4.53 |
>10,000 |
[8] |
|
Ethanol/water |
Faujasite |
0.89-1.59 |
130-360 |
[8] |
|
Methanol/water |
A type |
0.57-3.50 |
2,100-5,700 |
[8] |
|
2-Propanol/water |
A type |
1.76 |
10,000 |
[8] |
|
Ethanol/water |
A type |
2.1 |
2,140 |
[14] |
|
Methanol/water |
A type |
1.8 |
140 |
[14] |
|
Methanol/water |
Silicalite |
0.12 |
12 |
[43] |
|
Methanol/water |
ZSM-5 |
0.10-0.30 |
13-49 |
[43] |
|
Ethanol/water |
ZSM-5 |
0.05-0.11 |
2.1-29 |
[43] |
|
i-Propanol/water |
ZSM-5 |
0.03-0.06 |
0.9-16 |
[43] |
|
t-BuOH/water |
Silicalite |
3.5 |
144 |
[44] |
|
t-BuOH/water |
A type |
1.5 |
16,000 |
[44] |
|
t-BuOH/water |
Polymeric |
0.5 |
3,615 |
[44] |
Besides, membrane separation between alcohol and water usually exhibits quite high selectivities because of the strong affinity between zeolites and water. Fruitful results in this area have been achieved in recent years and are summarized in Table 2.
Mitsui Engineering and Ship-Building Co. first installed a commercial plant using 16 zeolite A tubular membrane modules and produced 600 L/hr of dehydrated solvents (ethanol, 2-propanol, acetone, etc.) containing less than 0.2% of water from feeds at 120°C containing 10% of water.[8] Shah e al.[14] explored the commercial zeolite NaA membranes for the pervaporation separation of binary solvent/water systems in a broad range of concentrations and temperatures. Tuan et al.[43] separated liquid mixtures by pervaporation using metal ion-substituted ZSM-5 zeolite membranes. Their results indicate that substituted ZSM-5 membranes had higher methanol/ water separation factors than silicalite-1. The B-ZSM-5 membrane had a methanol/water separation factor of 49. The separation factors and fluxes in pervaporation through substituted ZSM-5 membranes (except Ge-ZSM-5) were in the order methanol > ethanol >propanol. Gallego-Lizon et al.[44] compared the commercially available polymeric, microporous silica and zeolite (NaA type) membranes by pervaporation with respect to the dehydration of binary mixtures of t-butanol and water. They showed that the microporous silica membrane resulted in the highest flux (3.5 kg m~2 h~and the NaA zeolite membrane exhibited the highest selectivity (16,000).
ZEOLITIC MEMBRANES FOR MEMBRANE REACTORS
Zeolite membranes are thermally stable and well adapted to high-temperature applications because they can be used as separators only and/or as active contactors if they are catalytically active. The use of zeolite membranes in reactors has many advantages in terms of the several unique functions: to separate the products formed, to remove inhibitors, or to add reactants in a variety of reaction scenarios. In addition, the zeolite materials that constitute the membranes often have intrinsic catalytic properties, which makes it possible to conceive reactors where the membrane itself performs the reaction and separation functions. Integration of reaction and separation at the microscopic level has numerous potential advantages in applications, such as the increase of selectivity in situations where a short-lived valuable intermediate product is generated. It has demonstrated an increase in conversion of the reactants and, in some situations, increase in selectivities. Membrane reactors are now widely used in reaction processes.
Generally, there are two configurations in the membrane reactor. The first is that the membrane performs the exclusive separating function, and does not take part in the reactions. The other is an integrated system with the membrane simultaneously performing the functions of separation and catalysis in the reaction. In the former case, the products formed on the catalyst must desorb, diffuse to the membrane surface, and transport to the opposite side. Obviously, this introduces a supplementary transport resistance compared to the case where reaction and separation are carried out in the absence of catalytic materials other than the membrane itself.
Membrane Reactors with Separation Functions
In this case, zeolite membranes are used as separation membranes and not as catalytic membranes. The use of zeolite membranes for the dehydrogenation of hydrocarbons is a typical example for this configuration. Ciavarella et al.[45] showed that the isobutene yields were increased to four times higher than that observed in a conventional reactor. The possibility of using membrane reactors to increase the removal of hydrogen in dehydrogenation reactions or in other processes producing hydrogen, such as decomposition (H2S, H2O) and synthesis gas production, will significantly promote the conversion of reac-tants. Illgen et al.[40] fabricated H2-selective, MFI zeolite membranes on a porous ceramic tube, which was used to support the packed bed inside. The conversion of isobutane dehydrogenation was increased by almost a factor of 2.
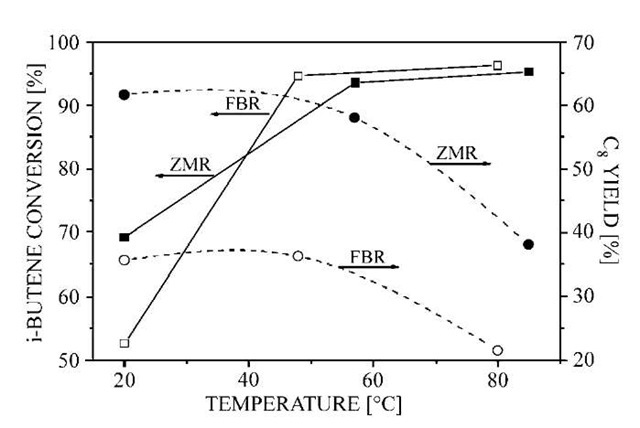
Piera et al.[46] investigated the use of an MFI (silicalite) zeolite membrane reactor (ZMR) in the liquid-phase oligomerization of i-butene. The membrane was used for the selective removal of i-octene from the reaction environment, thus reducing the formation of unwanted C12 and C16 hydrocarbons. Reaction experiments were then carried out over an acid resin catalyst bed located on the membrane tube side. Seen from Fig. 2, the ZMR (zeolite membrane reactor) produced a very significant increase in the selectivity, and as a consequence also in the yield of i-octenes (intermediate product in the oligomerization of i-butene), compared to a conventional fixed-bed reactor (FBR), while maintaining the similar i-butene conversion.
Salomon et al.[47] employed some different hydrophilic membranes (mordenite or NaA zeolite) to selectively remove water from the reaction atmosphere during the gas-phase synthesis of methyl-tert-butyl ether (MTBE) from tert-butanol and methanol. This reaction was carried out over a bed of catalyst packed on the inside of this zeolite tubular membrane. Their results indicate that MTBE yields were obviously higher than the traditional fixed-bed reactor. Farrusseng et al.[48] applied three different porous ceramic membranes (microporous MFI zeolite, mesoporous SiO2, and meso-macroporous AlPO4 membranes) to distribute O2 for the partial oxidation of C3H8 in an inert membrane reactor. The performance of the three membranes as O2 distributors were evaluated by their ability 1) to control the O2 partial pressure in the tube side and 2) to limit the back-diffusion of C3H8 to the shell side by application of a transmembrane pressure. The AlPO4 membrane was shown to be the most efficient membrane as a porous O2 distributor. Jafar et al.[9] applied zeolite A membranes to the esterification of lactic acid with ethanol to give ethyl lactate. Experimental results demonstrated the removal of water by pervaporation or vapor permeation enhanced the yields of ethyl lactate by about 20%. Liu et al.[49] applied the NaA membrane in a membrane reactor (NiO/La2O3-g-Al2O3 as catalyst) for CH4/CO2 reforming. CH4 and CO2 conversions, and CO and H2 selectivities were significantly higher than the values observed over a traditional fixed-bed reactor by more than 10%, especially for the increase of CO2 conversion by 30%.
Fig. 2 Comparison of ZMR (zeolite membrane reactor) and FBR (fixed-bed reactor) performances. The i-octene yield as a function of temperature.
Membrane Reactors with Combined Separation and Catalytic Functions
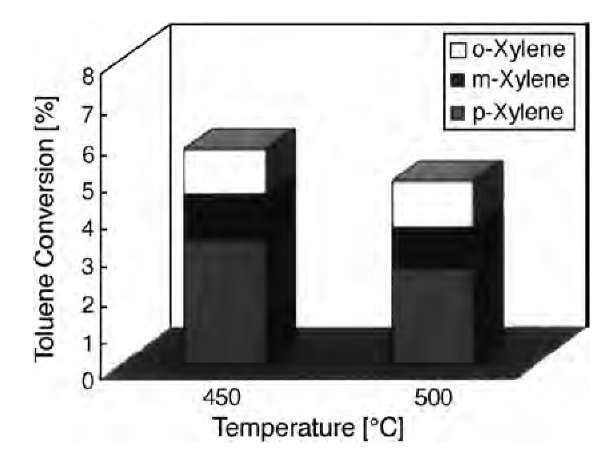
Zeolite membrane, with its intrinsic or incorporated catalytic properties, can be applied in zeolite membrane reactors, combining the reaction and separation functionalities at the same time. Oxidative dehydrogenation of alkanes is a good case in point. The potential use of catalytic membrane reactors in this case appears to be of particular interest for 1) controlling the oxygen feeding to limit the highly exothermic total combustion and 2) improving the contact between the reactant and the catalytic sites. Immobilizing transition metal ions in zeolites by ion exchange or by incorporation into the lattice leads to stable, isolated, and well-defined, redox-active catalytic sites. Julbe et al.[22] tested the oxidative dehydrogenation of propane on V-free MFI membranes and V-MFI zeolite membranes. Both MFI and V-MFI membranes were found to produce propene with about 40% selectivity but with higher O2 and C3H8 conversions for the V-MFI. Hasegawa et al.[50,51] applied noble metal-impregnated Y-type zeolite membranes for the selective oxidation of CO, which was present at concentrations of several 100 ppm in a stream of hydrogen. Ciavarella et al.[52] separated H2 and isobutene mixture using composite alumina-MFI-zeolite with a separation factor of 25 at 723 K, a typical temperature of the isobutane dehydro-genation in membrane reactor, which indicates a good commercial prospect. Takata et al.[53] applied the ion-exchanged H-type MFI zeolite membranes to the alkyl-ation of toluene with methanol. A p-xylene selectivity of 80% were attained using H-MFI zeolite membranes at 450-500°C, as illustrated in Fig. 3, providing the possible application of an MFI zeolite membrane for use in a catalytic membrane reactor (CMR).
Fig. 3 Toluene conversion
Bernal et al.[54] used a catalytically active zeolite membrane to shift the reaction equilibrium by selective water permeation during ethanol esterification. The H-ZSM-5 membrane used in their work had sufficient catalytic activity to carry out the esterification of ethanol with acetic acid, and at the same time was selective for water permeation. As a consequence, the reaction and separation functions could be very efficiently coupled, and the conversion obtained (63.1%) at the same feed rate and catalyst loading was greater than in conventional fixed-bed reactors (49.4%), or in reactors where the zeolite membrane was kept separated from the catalyst (56.9%). Zhang et al.[23] fabricated the crack-free Fe-MFI zeolite that was used for the conversion of ethylbenzene to styrene and obtained 15% of conversion increase over the conventional fixed-bed reactor.
CONCLUSION
Successful synthesis of high-quality microporous zeolite membranes has opened up new fields of membrane applications in environmental separation and reactions. Some separation applications such as H2 from mixtures and water/alcohol separation have great commercial potential. Zeolite membranes permit the integration of reaction and effective separation in a single stage. Some reactions have been tested in zeolitic membrane reactors and showed promising results. Extending the catalytic zeolite membrane to more reactions is currently the focus of many industrial and research groups around the world.
However, many challenges exist in zeolite membranes. A typical example is the low flux through the zeolite membrane because of the fact that relatively thick zeolite layers on the order of a few micrometers to 50 pm are necessary to ensure a pinhole-free and crack-free zeolite layer. Decreasing the membrane thickness while keeping its compactness will dramatically increase its flux, thus resulting to more throughputs in practical applications. Some researchers have reported some significant advances in increasing the gas flux through the membrane by reducing the membrane thickness or by fabricating the membrane with higher surface area. However, reproduc-ibility for thick zeolite membrane fabrication is always a challenge. In this regard, the search for optimal zeolite membrane materials and novel synthesis methods for improved chemical and structural stability will require continuing efforts of researchers.
Many catalytic processes of industrial importance involve the combination of high-temperature and chemically harsh environments, two factors that strongly favor zeolite membranes. They can be integrated in broad arrays of membranes in membrane reactors for separation and reaction applications, ranging from biochemical through environmental to petrochemical applications. The zeolite membranes can be employed for the permeation of the products to shift equilibrium limitations or the distribution of reactants for the control of concentration of selected species, or to carry out catalysis and separation at the same time. Hence conversion enhancement can be significantly achieved in several reaction processes such as hydrogenation, dehydrogena-tion, and esterification. Therefore the employment of zeolite membrane reactors for several industrial applications is envisioned. However, scale-up problems are still the main limitations of this technology, in particular that large, crack-free zeolite membrane surface areas are required for membrane reactors in industrial application. This area requires major research and development efforts to take the technology to the industrial application stage.