Introduction and Overview
Within the various domains of criminalistics, the investigator compares traces found at the scene of a crime with those recovered from a person, tool or place. By contrast, the work of the fire investigator is not purely comparative in nature; extensive practical experience of the examination of as many fire scenes as possible combined with a sound scientific knowledge, especially in the areas of physics and thermodynamics, is paramount to the success of the investigations. These two criteria enable him or her to identify the origin of the fire and determine its cause despite having to work on sites that have been destroyed by the fire and further disrupted during the extinguishing process.
This article explores: the relevant background areas of physical thermodynamics; the role of thermodynamics in fire investigation; fire ignition and propagation; thermodynamic classification of ignition sources; and the phenomena of smouldering and flaming combustion.
Physical Thermodynamics: the Relevant Background
Before considering the role of thermodynamics in fire investigation, it is essential to remember that the fundamental subject under scrutiny is concerned with the transformation of a macroscopic system which is dependent on one of the basic elements of physics: temperature. It is also important to under- stand that an intensive thermodynamic variable is one which is dependent on the amount of a chemical substance in the system, whereas an extensive variable does not.
Physical systems
A physical system is defined as that part of the universe under the influence of thermodynamics which has been identified for study. It can be:
• isolated: no exchange of heat, work or matter with the surroundings is possible. The system is said to be ideal and can be assumed in various situations;
• closed: exchange of heat and work, but not matter, is possible with the surroundings;
• open: exchange of heat, work, and matter is possible with the surroundings.
Thermodynamic principles
Two of the fundamental laws of thermodynamics can be described in the following manner:
The first law of thermodynamics All systems possess an internal energy U, which is a state variable, signifying that U is independent of the history of the system. When a system undergoes a change from state 1 to state 2, the difference in thermodynamic energy, AU is:
![]()
While respecting the principle of the conservation of energy, the AU of a closed system can be expressed as:
![]()
where AQ and AW are, respectively, the applied heat and work.
For an isolated system U is constant; in keeping withthe conservation of energy, U1 is therefore equal to U2. There is no energy change between the system and its surroundings therefore:
![]()
The second law of thermodynamics This law concerns change and entropy. Entropy, S, is an extensive state variable which denotes the degree of disorder of the system. During reversible changes, the system and surroundings are in constant equilibrium. When considering such a change from a state 1 to a state 2, the entropy of a system is:
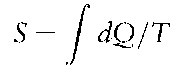
irrespective of the pathway taken.
However, energy dissipated in the form of heat is not an ideal process; it is spontaneous and irreversible
which consequently means that the entropy of such a system increases.
The Role of Thermodynamics in Fire Investigation
An understanding of fundamental thermodynamics is important in fire investigation in considering possible ignition processes.The most important factors are identified below.
Temperature
Temperature is an intensive state variable which can be measured for any given system by the use of a thermometer.The Celsius, or centigrade, scale of temperature is governed by the triple point and the boiling point of water.At 0°C, under standard pressure, water exists simultaneously in solid, liquid and gaseous states; at 100°C only the existence of the liquid and gaseous states is possible.There are two other temperature scales which are extensively used: Kelvin (K) and Fahrenheit (F).The conversion formulae between the three scales are as follows:
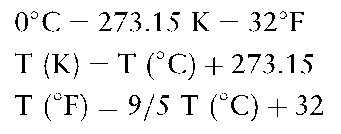
At any given atmospheric pressure there are certain temperature values, which are specific to a particular substance, that indicate whether the ignition of this substance is possible.
• Flash point: the lowest temperature at which a sufficient quantity of vapor is produced above a solid or liquid which can be ignited by a flame, spark or localized heat source.At this temperature combustion does not endure;
• Point of inflammation: the lowest temperature at which the production of flammable vapors above a solid or liquid is such that a flame, spark or other localized heat source will cause their ignition; combustion and its accompanying flames are sustained;
• Point of auto-ignition: the lowest temperature at which a solid or liquid will spontaneously ignite without the presence of an additional heat source (flame, spark or other).
Heat
Heat is a form of energy which has a number of effects on a system.lt can be generated by the transformation of energy, be it mechanical, electrical, chemical or other, into calorific energy.A temperature gradient exists between two systems at different temperatures, along which calorific energy (heat) is transferred in order to reach a state of thermal equilibrium.
The fundamental unit of calorific energy is the Joule (J); however, a more widely recognized terminology is the calorie (cal) or kilocalorie (kcal).These units are defined as the quantity of heat required to raise 1 g, or 1 kg respectively, of water from 14.5°Cto 15.5°C.
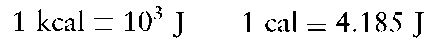
Physical properties
Heat capacity, C, is the heat transferred to a body in order to raise its temperature by one degree (K):
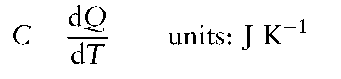
Specific heat capacity, c, is the heat capacity of 1 kg of a substance which, in general, is dependent on temperature:
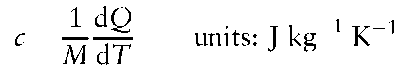
One of the fundamental laws of physical chemistry is the Ideal Gas Law:
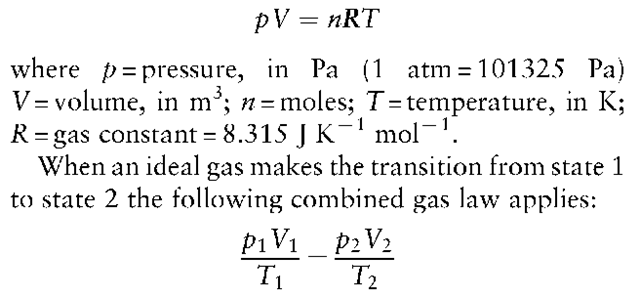
This expression can be used for real gases on the understanding that the data obtained will only be an indication of and not the exact values desired.
Fire: Ignition and Propagation
Fire is an oxidation-reduction (redox) reaction accompanied by the production of heat and light.The two chemical requirements are an oxidizing agent (or combustive agent) – usually the oxygen in air – and a reducing agent (or fuel), which must be in a gaseous state in order to mix with the air.An activation energy, always in the form of heat, is required to ignite the gaseous mixture and combustion is maintained by the calorific energy produced during the exothermic reaction.
Activation energy
The limit of flammability of a gas (or gaseous mixture) is expressed as a percentage which indicates the proportion of gas present in the air.The ignition of a homogeneous mixture of air and a gas is possible when the lower limit of flammability has been attained; however, ignition is impossible above the upper limit of flammability as the air is too saturated. Understanding and establishing the activation energy that is required for ignition of a gas/vapor (other than autocombustion) is fundamental to fire and explosion investigations.The scene examination is often complex as several calorific energy producing systems can be present, of which some could be weak heat producers and others situated some distance from the combustible material.In any situation, the means of ignition has to originate from one of these sources. In order to eliminate each ignition possibility until left with the most probable, a second factor must be taken into consideration: the thermal or calorific power.
Thermal power and reaction temperature
The thermal or calorific power of a system is the calorific energy produced over a period of time.The investigator can only estimate the thermal power which is at the origin of and aids the development of a fire.The relationship between the thermal power of the heat source, WT, and that dissipated in the surrounding environment, wT, constitutes the thermo-dynamic foundation of the whole investigation.This can be seen from the following equation derived from the Arrhenius expression:
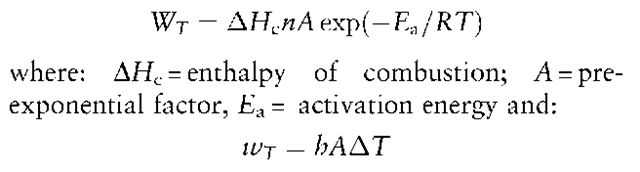
where: h = convective heat transfer coefficient; A = surface area.
The thermal power of a heat source is exponentially dependent on temperature, and the only circumstances when a body will not emit heat is at absolute zero temperature (0 K); W =0 if, and only if, T =0.
This observation is important in that any system not at T =0 K will produce calorific energy.If the energy dispersion into the surrounding environment is restricted, the system’s temperature will inevitably increase.Ignition frequently occurs where the temperature of a combustible material has been augmented to the point of autocombustion.
The possibility of a given heat source (spark, flame, fermentation, etc.) igniting a specific material (wood, insulation, paper, etc.) in a certain environment (quasi-isolated, closed or open) must always be considered before the source can be eliminated from the investigation.However, calculations of WT and wT for real systems are impractical and inexact, except for certain types of heating; instead, the essential factor that should be taken into consideration when examining possible ignition sources is the influence of WT and wT on the system.Therefore, the first step of a fire/explosion investigation is to locate the place where the activation energy was produced; in other words, to determine the foyer, or seat, of the fire/ explosion.Once a potential heat source has been identified, the implications of the thermal power produced and the heat dissipated in its immediate vicinity should be estimated.This enables the investigator to verify whether or not the proposed source is a plausible source of ignition of the material situated at the (presumed) foyer.
Combustion speed
The phenomena of smouldering fires, flaming fires and explosions differ only in their rate of combustion.
• Smoldering is a flameless combustion involving the slow oxidation of a porous combustible material, which occurs in an environment where oxygen can easily penetrate yet is sufficiently isolated so that heat dissipation to the surroundings is minimal.
• The speed of combustion of a flaming fire can vary enormously.The chemical reaction occurs without a perceptible pressure increase within the immediate vicinity.
• An explosion is an extremely rapid combustion. When occurring in a confined environment it is accompanied by a considerable augmentation of pressure.The reactants are present within their limits of flammability and are usually in a gaseous state; however, very fine droplets of liquid, such as those produced by an aerosol, or fine powders/dust can also combust explosively.After ignition, the entirety of the explosive material undergoes the exothermic reaction of combustion almost instantaneously; the ambient air subsequently expands very quickly resulting in a pressure increase in the immediate environment.
Thermodynamic Classification of Ignition Sources
Fires can be classified according to their ignition source, whether due to a spark, naked flame or localized heat source.
Heating
Heating is a transfer of calorific energy to a physicochemical system without the presence of a spark or flame.If the system generates heat itself to augment its temperature to the point of inflammation and subsequently to autoignition this is known as spontaneous combustion.With the exception of this latter form of heating, heat is transferred to the material via the methods outlined below.
Conduction This is the mode of heat transfer without displacement of matter, due to electronic movement within a solid.Where two faces of a solid are at different temperatures, T1 and T2, the heat flux, q’, through the material is:
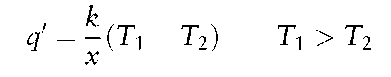
where: x = thickness of material; k = thermal conductivity.
If a steady state heat flux traverses two (or more) layers of material:
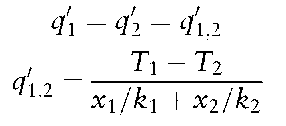
Convection Convection occurs in liquids and gases and is a transfer of heat via mechanical movement due to changes in density.This process can, however, transfer heat to a solid.For example, if a solid surface of area A is in contact with a liquid/gas at temperature T, the heat flux, q”, transferred to the surface is:
![]()
where: h = convective heat transfer coefficient; A =surface area.
Radiation Electromagnetic radiation in the visible and infra-red regions is radiated from all bodies with a temperature above absolute zero.When a system is in thermal equilibrium its components emit and absorb radiated heat at the same rate.Although this radiation is rarely quantified it is important to note that:
• radiation is the prevalent mode of heat transfer between flames and combustible material; it is therefore the principal cause of vaporization and consequently the main means of the ignition of fresh material;
• before suspecting human intervention after a large fire where several foyers have been identified, it is essential to verify that the separate ignition sites are not a consequence of thermal radiation.Fire may have spread in this manner from one combustible material to another despite the lack of an apparent physical link.
When a body has two identical surface areas, A, respectively at temperatures T1 and T2, a heat flux, /, flows from surface 1 to surface 2 if T1 > T2:
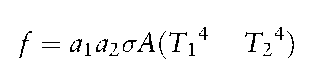
Where: a = heat absorption or reflection factor; a = Boltzmann constant.
Heating appliances When a heating appliance is identified as being the origin of a fire, any of the three modes of heat transport can constitute the transfer of calorific energy to the combustible ma-terial.As the correct use of these devices is for the generation of heat, the cause of the fire has to be a result of an inappropriate usage or a malfunction of the apparatus.
Electrical sources Fires of electrical origin are often complex to investigate and difficult to prove.Heat is produced when an electrical current, J, passes through a conductor with resistance, R, over a time period, t, according to Joule’s Law:

Friction The generation of heat by the friction between two objects is proportional to the speed at which the two bodies move against each other and their respective weights.The nature of the surfaces in contact, which is indicated by the friction coefficient, will also have a bearing on the quantity of calorific energy produced; those with a high friction coefficient generating more heat.
Sparks Material sparks Material sparks are produced when solid particles with a mass of milligram magnitude are emitted into an open system existing at high temperature.These tiny fragments of incandescent or burning matter can constitute the origin of a fire if:
• when smoldering, the distance between the source and the combustible material is very short; due to their miniscule mass the particles lose heat rapidly;
• the spark enters into contact with a highly flammable material or one with a small heat capacity in which a smoldering fire can manifest.
A spark of mass m, and heat capacity c, emitted with a temperature T2, into an environment at temperature T1, produces heat, Q, according to:
![]()
For example, when grinding a metal, the metallic fragments produced are of milligram mass, which generate an approximate heat of Q«1.3 J; this is of large enough magnitude to ignite a flammable gas/ vapor.Another example is the fusion of a metal/metal oxide when welding; the resulting droplets have a diameter of 2-5 mm and generate heat between the values of: 37 < Q < 578 J.The first value is more than sufficient for the ignition of a gaseous mixture, whereas the second can initiate the combustion in a nearby combustible material such as plastic isolation or wood, provided that it is within the boundaries of the quasi-isolated system.
Electrical sparks and arcs In the space separating two charged objects, there is an electrical field.If an avalanche of electrons occurs due to this field it is visible (to the naked eye) as a spark.Because of its short lifetime, an electric spark produces a relatively small amount of heat; nevertheless, the millijoules of energy generated can ignite a homogeneous air/flammable gas mixture resulting in an explosion.
The difference between an electrical arc and spark is the duration of the electrical discharge: that of an arc being markedly longer than a spark, which can be said to be instantaneous.Logically, it follows that the energy produced by an arc is of greater magnitude and can subsequently inflame a combustible material rather than uniquely gaseous mixtures as for a spark.
The above phenomena will occur when in the following conditions.
• A defective contact (of a switch, relay unit, etc.) in an electric circuit, with or without a coil, will result in an increase in resistance and will thus generate heat.This default can also occur with the use of adapters and extension cables.
• In the presence of an electrolyte (water, humid air, etc.), a defective insulation of a cable will enable the passage of an electric current between two conducting wires at different electric potentials. An augmentation in resistance will subsequently generate calorific energy at a precise point which can be sufficient to ignite the insulation or a combustible material in close proximity.
Smoldering
In a smoldering solid the heat conduction is difficult to calculate, as are the speed and propagation of combustion.This type of fire obviously still requires an ignition source, however the thermal power of the source can vary enormously.For example:
• after most fires, smoldering is the natural progression of flaming combustion.This latter form of combustion can be reinitiated when a great quantity of smoldering material is remnant after a large fire.This eventuality only applies to one aspect of fire investigation: that where flames are rekindled from smoldering remains, even after extinction by the fire brigade; • when the heat source is weak, smoldering combustion commences at the surface of the material.In this case the only conceivable progression of combustion is to the interior of the material, otherwise self-extinction will occur.
Smoldering combustion is self-maintained if an equilibrium is established between the thermal power of the exothermic (combustion) reaction and that dissipated into the surroundings:
![]()
If the difference, AW, between the former thermal power values is small, the fire will neither self-extinguish nor reach an open flame state; the smoldering combustion will simply continue to burn fresh material with a random progression that is due to the inhomogeneous environment.In order to maintain a constant value of AW, or one that varies within a very narrow range, the oxygen supply must be adequate; nevertheless, it must be noted that even in an oxygen-deficient environment (proportion of O2 in the air <16%), it is possible that this type of combustion will be sustained.
Smoldering material will burst into flames if there is sufficient heat build-up and enough oxygen avail-able.For example, smoldering combustion will progress to the flaming stage if the burning material enters into contact with air.A pertinent example of this is when the exterior surface temperature of a material is increased by heat transferred from internal smoldering combustion; when the surface, which is in contact with the ambient air, reaches the point of inflammation flaming combustion will result and will spread at a rapid rate.
Flames
If a combustible material is exposed to prolonged contact with a flame its temperature will eventually reach the point of inflammation and ignition will occur.The resulting flames will subsequently raise the temperature of the surrounding material which will also attain its inflammation point and start to burn.The propagation of fire by direct flame contact is a natural progression which does not require further explanation.
Hot gases produced by burning material are propelled upwards.Due to their inferior density the buoyancy forces within the smoke plume cause the hot gases to rise with a speed that is dependent on the temperature of the fire.At high temperatures, for example directly above the flames, the gases are dispersed vertically very rapidly and have little sideways diffusion into the ambient air.This effect diminishes as the ambient temperature decreases, thus the gases have greater horizontal convection the further they are from the flames.They can therefore be seen to rise in the form of a three-dimensional ‘V (an inverted cone).This plume of hot gases transports soot particles which are deposited on vertical nonflammable supports; the characteristic triangular pattern of these deposits can often be used to locate the seat of a fire.
The smoke resulting from a fire contains combustion products and small unburned residues which are, by definition, nonoxidized vapors that are transported by the diffusion of the other hot gases.These vapors can accumulate in enclosed spaces and spontaneously ignite, under the correct conditions, even at considerable distances from the original foyer.
Fire spread can often be deduced with relative ease if the initial heat source has been identified and there is evidence of the presence of combustible material throughout the entire burned area.However, when justifying a source of ignition, problems can arise when the heat source is found to be separated from the combustible material by nonflammable material such as walls, floors and ceilings.
With an open fire, the vertical flame movement and the air supply are practically limitless.At the exterior of a stone or brick building, a fire situated at ground level can extend to roof height.If the roof is constructed from combustible material such as wood or thatch, it will eventually catch fire (due to the prolonged contact with the flames and hot gases) even if separated from the seat of the fire by several floors of nonflammable material.
If the fire is restricted by a wall or other incombustible barrier, the supply of air may well be limited.The flames will consequently spread towards the direction of the air source.When vertically extending flames are hindered by the ceiling of a room, the plume of hot gases is redirected along its surface.The horizontal spread can be of considerable distance as the area directly below the ceiling is at a highly elevated temperature, thus sustaining the capacity of the gases to ignite fresh combustible material.Therefore:
• if the entrainment of air and the diffusion of flammable gases/vapors in the vertical part of the flame is sufficient, there will be limited flame spread: combustion will occur at ceiling level;
• with a low ceiling or a vapor-rich smoke plume, the hot gases will spread out beneath the ceiling and the flames can spread to material situated some distance from the original fire seat.
It must be remembered that a corridor, ventilation shaft or other form of passageway can aid the propagation of fire between rooms, apartments or even separate buildings.
Conclusion
This article does not attempt to list each and every possible heat source capable of initiating a fire. Instead it indicates the most significant sources and analyzes them thermodynamically, consequently highlighting the role of thermodynamics and that of fundamental physics in verifying the conclusions of the fire investigator.Nevertheless, it must be noted that numerical values are rarely calculated when considering the various exchanges of heat or the laws of thermodynamics, as the fires under investigation were not performed in the laboratory under controlled conditions.The equations and mathematics are simply used to give an indication of the magnitude of the process in question.The work of the fire investigator is therefore to identify the origin of a fire and to report its cause by exploiting an extensive practical experience and intricate knowledge of the laws of physics and chemistry.
