Introduction
To reveal a crime the forensic scientist looks for marks a felon may have left at the site of the event or brought with him. These are often comprised of agents suitable for chemical analysis, e.g. drugs seized by the police or present in a body fluid, residues of arson accelerants or of explosives, poisons in a dead body to name a few examples. A main problem is that the chemist does not know which compounds to look for in a wide range of possible ones with different physicochemical properties. He has to search for the ‘general unknown’, a circumstance that requires not only solid professional skill but also good test tools. One of the chemist’s main analytical aids to solve forensic problems by revealing the ‘general unknown’ is the use of mass spectrometry (MS).
On the basis of experiments on the behavior of charged particles in electrical and magnetic fields, J. J.
Thomson built the first mass spectrometer known as the hyperbola spectrograph. Since its advent in 1913 several types of mass spectrometer have been used as the most versatile apparatus in diverse analytical situations to detect and identify virtually any analyte molecules that can be converted to the gas phase at a reasonably low pressure. Also, the method provides an excellent means of finding out the molecular weights and gaining structural information on ana-lytes, even of those larger than 10 000 Da.
In short, MS is a method by which one generates charged molecules and molecular fragments, and measures each of their mass, or more correctly, the mass/charge ratio. The uncharged gas molecules, which are introduced into the mass spectrometer, move around randomly in space, and to control their motion they have to be ionized, i.e. a charge is added to the molecules. To obtain structural information about the molecules, energy in excess of the ionization is supplied to break some of the covalent bonds holding the molecules together.
Even though mass spectrometers work differently to reach these goals, they have some general operating concepts in common. These are shown in Table 1 and consist of the inlet system, ion source, mass analyzer, mass detector, data collecting system, and data interpretation scheme.
Inlet System
The inlet system is used to introduce the sample into the mass spectrometer, to convert it into the gas phase, and to reduce its pressure before ionization. Forensic samples are often impure, and the analytes, therefore, have to be separated from the matrix before insertion into the mass spectrometer. This is done by chromatographic methods, i.e. thin layer chromatography, gas chromatography (GC), or high-pressure liquid chromatography (LC). After thin layer chro-matography the cleaned-up sample is introduced into the mass spectrometer by the direct insertion probe. The specimen to be analyzed is put on a metal rod,which is inserted into the mass spectrometer and then heated to evaporate the sample close to the ion source. Provided that the contaminants in the forensic samples have boiling points which differ sufficiently from that of the analyte, some separation may yet be possible by slowly increasing the temperature of the rod. The method is rather imprecise making it inappropriate for quantitative study; the technique of direct inlet insertion is therefore seldom used for MS analysis of forensic samples.
More suited is the use of the inlet system as an interface between a chromatographic device and the mass spectrometer. By this technique the analytes are separated from one another and from the contaminants by either GC or LC, and the effluents from the column flow directly into the mass spectrometer. The on-line coupling of a gas chromatograph to the mass spectrometer was the first interface to be developed. It functions in a quite different way than the LC-MS interface, which for forensic aims is less used than the GC-MS interface.
The LC-MS interface has a number of problems. The main reason for this is the fact that the eluate from the column is a liquid, and on changing to a gas for MS analysis it expands, thus creating an extra burden on the vacuum system for reducing the pressure in the instrument. Moreover, the effluents often carry polar, heat-labile substances that may taint the ion source when they are vaporized.
With the most often used inlet of the LC effluents, i.e. the thermospray interface, these are forced through a pinhole leak to form a jet of liquids, which is heated to vaporize the solvents in the aerosol. The mist of droplets in the gas phase, which becomes void of solvents, carries along with a charge from any ions initially present in the solution the less volatile analytes directly into the ion source of the mass spectrometer to become fit for analysis.
GC-MS was devised about 25 years ago, and in those days the GC separation was done with packed columns. Since small amounts of the analyte are then eluted from the column in a large volume of carrier gas at atmospheric pressure, whereas the mass spectrometer works at about 10 ~ 3 Pa, the interface has both to lower the pressure of the sample before it enters the mass spectrometer, and also to concentrate the analyte. This is achieved with a molecular separator, in which the effluent from the gas chromato-graph, made up by the carrier gas of lower mass than that of the analyte, is pressed through a capillary with a tight outlet into a nearby capillary with a wider orifice. By this procedure the lighter molecules are more readily lost into the vacuum area, whereas the heavier analyte molecules move through the second capillary into the mass spectrometer, which they reach after having been enriched.
Table 1 Operating concepts of mass spectrometers
| Inlet system | Ion source | Mass analyser | Detector | Data collecting | Data |
| system | interpretation | ||||
| Direct inlet | Electron impact | Magnetic sector | Electron | Full scan mode | Library |
| instrument | multiplier | searching | |||
| Gas | Chemical | Quadrupole | (Time-of flight)a | Selected ion | (Artificial |
| chromatography | ionization | instrument | monitoring | intelligence) | |
| Liquid | (Desorptive | Ion-trap detector | Negative ion | ||
| chromatography | ionization) | Tandem MS | detection |
Today in most gas chromatographs, however, the packed column is replaced with a capillary column to do the separation. This has to a great extent made the problem of introducing the analytes into the mass spectrometer easier. Since the flow rates are much lower in a capillary than in a packed column, the whole volume of the effluents can be let into the mass spectrometer without any losses in the interface. To do this, the end of the GC capillary is close to the ion source of the mass spectrometer. With this system there are two things the user must keep in mind. One is that with a magnetic sector or a quadrupole instrument a vacuum pump must be used with enough capacity to guard an adequate pressure in the ion source. With an ion-trap, which also needs a vacuum pump, this aim is reached by fine tuning the flow rate through the capillary. The other factor is to keep the interface at a raised temperature to avoid analyte condensation in the liner.
Ion Source
When the analytes enter the mass spectrometer’s ion source these are submitted to ionization. The main aim of this step is to put a charge on the molecule, a process needed to enable a control of the molecule path in a magnetic or oscillating electric field. Another goal is to break some of the chemical bonds of the molecule by putting energy into it.
The simplest ionization method is to impinge the analyte molecule (M) with a beam of energetic electrons (e~), a process referred to as electron impact (EI), and which results in the formation of a radical cation with an odd number of ions (M+.) according to the following equation.
![]()
As seen the analyte molecule has lost an electron and become a particle with a positive charge, thus allowing it to be separated in a magnetic or oscillating electric field based on its mass and number of charges.
The energy of the electrons is generally set at 70 eV, an optional value chosen because it is high enough to exceed the ionization energy at about 10 eV, and to split the molecule by breaking its chemical bonds.
For the identification of an analyte it is often of utmost importance to know its molecular weight. However, this information is generally not gained by ionization with electron bombardment; the process often results in a complex fragmentation of the molecule, making the deduction of the original substance mass difficult. To overcome this problem a milder ionization method has been devised, i.e. chemical ionization (CI). In this process the analyte molecule reacts in a gas phase with a proton donor formed by EI of a reagent gas, e.g. methane. The reactions occur according to the following.
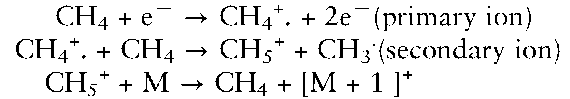
By accepting a proton and taking up a charge, the analyte molecule, thus, reacts with a secondary ion formed by EI of the reaction gas, methane. The process now allows the analyte molecule to become available for MS analysis and to show up in a mass spectrum with its protonated variant as the most abundant peak.
Mass Analyser
The analyte molecules, which have become ionized, may now be moved apart from one another in a magnetic or oscillating electric field. Even though the main object is to analyse the fragments based on their masses, it is the mass/charge (mlz) ratio that forms the ground for the separation process. This means that a fragment with m/z = 200/2 will have the same trajectory as a fragment with m/z = 100/1, and they may, thus, not be distinguished from one another. Fragments with two charges are, however, rare, and in practice it is the mass of a fragment that one generally regards as the controlling factor of its movement in a magnetic or oscillating electric field.
Magnetic sector instrument
The first commercial mass spectrometer was a magnetic sector instrument. Charged fragments or molecules, which are accelerated and ousted from the ion source, tend to adjust to orbital movements when allowed to travel in a magnetic field aimed perpendicular to the flight of the ions. The trajectory of a fragment with a given m/z value, thus, depends on the acceleration voltage and the strength of the magnetic field. This means that only those fragments, which have a m/z value to balance the centripetal force of the magnetic field and become stable at a given acceleration, will travel through the flight tube and reach the detector, whereas the unstable ions will adhere to the path wall.
The fragments exit the flight tube through a slit, whose width determines the analytical resolution and the sensitivity. Awide opening favors a high sensitivity but low resolution. Asmall aperture, on the other hand, gives a high resolution but low sensitivity, and a reason for this is a certain spread of fragments with the same m/z value but with different kinetic energies. These fragments will, thus, not be recorded. To compensate for such a loss one may insert either before or after the magnetic sectors, a device to focus the fragments with the same m/z value, i.e. an electrostatic analyzer. This procedure forms the basis for the double focusing mass spectrometer.
Quadrupole instrument
Another type of mass filter is the quadrupole. Its ability to separate fragments with different masses is based on the fact that, when ions travel in a beam of an oscillating electric field, their trajectories become influenced. The quadrupole mass filter is made up of four parallel rods, about 10 cm long and 1 cm in diameter, which in cross-section are arranged to form a square box with a gap in between the four bars. The fragments are allowed to travel in this space along the rods, where they are accelerated in the oscillating electric field set up by dc and ac potentials applied to the rods. At a certain ac and dc potential, ions with a specific m/z value will become stable and oscillate in a fixed path through the quadrupole to reach the detector to be recorded. The fragments with m/z values that do not suit the applied potentials will become unstable, and, as in the magnetic sector instrument, not reach the detector but stick to the rods.
Ion-trap detector
Athird type of mass filter is the ion-trap detector (ITD). Like the quadrupole, the ITD operates on the concept that when ions travel in an oscillating electric field their trajectories become influenced by the wave frequency. Unlike the quadrupole, however, the ITD uses a closed cavity in which the electric field is set up by the dc and ac potentials applied to a ring electrode and an end cap of the cell. The molecules enter the cell where they are ionized by an electron beam, and under the control of the applied electric field the ions are forced to move in an orbit within the space of the cell. When the ac or dc potential is changed, the motion of some ions becomes unstable, and 50% of these are then ousted from the cell through a hole in its bottom to be recorded by a detector. Unlike the quadrupole or magnetic sector instrument in which the stable ions are registered, the ITD, thus, looks at the unstable ions. This means, that compared with the quadrupole or magnetic sector system, the ITD offers a rather different analytical concept.
Tandem mass spectrometer
To gain a higher selectivity and also more information about the nature of an analyte, its analysis can be done by tandem mass spectrometry (MS/MS). Another main advantage of MS/MS is its ability to reduce the chemical background noise, and, thus to increase the signal-to-noise ratio for a detected peak. Although this method, as judged from the number of scientific papers reported, does not seem to be in common use for forensic analysis today, MS/MS will probably become the method of choice in the future. It may offer the forensic chemist a powerful tool for the analysis of the complex mixtures presented. In MS/ MS two mass spectrometers are linked in series to each other, in the early apparatus set up by two magnetic sector instruments. However, since these instruments are very bulky and high-priced, modern tandem devices are nearly all based on the use of quadrupoles or ITDs.
Adual quadrupole mass spectrometer actually entails three quadrupole units. In the first unit the incoming parent ions are separated from one another. In the second unit, which is charged only with radio frequency but not with dc the parent ions are taken to a common focus without any extra mass separation, and, similar to the mode of chemical ionization, split into daughter ions by collision with a gas let in from the outside. In the third unit the daughter ions are mass filtered as described above under ‘Quadrupole instrument’, and a fragmentation pattern in addition to that of the parent ion can be formed from each one of these.
Arather recent MS/MS development is the use of the ITD. To call the device for this approach a tandem mass spectrometer may, however, be somewhat deceptive. Even though the MS/MS analysis is done in three steps as described above for the quadrupole MS/MS, these are all carried out at the same site, i.e. within a single ITD cell. In the first step the parent ions, which are to be submitted to further analysis, are stored in the cell. Unlike the conventional ITD mode, when the target ions to be analyzed are the unstable ones, the MS/MS operation now saves the stable ones, whereas the unstable ions are discarded from the cell. In the second step the selected parent ions, moving in an orbital, are allowed to collide with a gas let into the ITD cell, whereby the daughter ions are formed. While traveling in the oscillating electric field, some of these become unstable at a specific change in the ac or dc potential applied on the ITD cell, and are then finally ejected to the detector to generate a daughter-ion spectrum. Since this MS/MS approach is carried out in a single compartment, rather than in three separate ones, there is a saving in cost.
Detector
In most instances, the separated ions are detected with an electron multiplier. Its task is to convert the charged fragments into electrons, amplify these, and transfer the electrical current set up to a wire for recording as a signal. Usually a so-called horn-type electron multiplier is used, since this variety of detector is the most compact and low-cost device. When the charged fragments enter the detector and strike the surface area of the horn, electrons are emitted. After acceleration of the electrons by an electrical potential difference in the horn, they in turn hit the surface and new electrons are formed, a process that is repeated over and over again to generate a cascade of progressively raised number of electrons. Usually the gain of emitted electrons is in the order of104-107 per ion entering the detector.
Data Collecting System
The MS test can be run in the full scan mode or in the selected ion monitoring (SIM) mode. In the full scan mode the mass analysis covers a range of m/z values, whereas in SIM a limited number of m/z values are selected for the experiment. When choosing between the two options, the operator of the mass spectrometer has to decide to what extent he is willing to trade sensitivity for selectivity or vice versa. This consideration is based on the kind of study, i.e. a search for the ‘general unknown’ or for the suspected agent. If no specific substance is expected to be present in the sample, he may be bound to screen for a number of candidates, e.g. for drug substances in a toxicology survey or for accelerants in an arson study. In such a situation the mass spectrometer is run in the scan mode, which means that the apparatus is set for recording a number of m/z values in a wide mass range. By this approach a number of unforeseen agents can be detected, but, unfortunately, at the sacrifice of sensitivity. On the other hand, if a specific compound is to be verified, the mass spectrometer can be set to focus on a single m/z value or a limited number of ions that are formed from the expected find in the sample. This is an analytical approach that aids a high sensitivity, but also yields only a narrow range of substances. The analytical condition the operator has to consider also depends on the type of mass spectrometer being used, i.e. the quadrupole, the magnetic sector instrument or the ITD. From the analyst’s point of view, the quadrupole and the magnetic sector instrument, which are so-called beam-type scanning mass spectrometers, offer rather small differences. They both operate on the principle of mass-selective stability. This means that ions with a small range of m/z values, conforming with the magnetic instrument’s given magnetic field strength and acceleration voltage, or with a quadrupole filter’s oscillating electric field, will be transmitted through the discriminating device into the detector. By this means the ions with stable trajectories become detectable, whereas those with the unstable ones are trapped on the magnet or on the quadrupole rods. Avital aspect of this operation mode is that the ionization and the detection occur, as a continuous process not separated in time, a circumstance that to a large extent affects the sensitivity. The reason for this is that the window of the stable m/z values is sequentially swept across the entire m/z range of interest, and the rate of the window width/entire m/z range, i.e. the duty cycle, is in most scanning tests only a fraction of 1%. More than 99% of the ions from the target agents are, thus, lost. Aduty cycle of 100% is yet possible with a beam-type instrument but only when run in a nonscanning mode, as in the SIM. Such an application, though, requires that the analyst know what he is looking for in the sample.
As described earlier the ITD monitors the ions with unstable trajectories, and, thus, operates according to the mass-selective instability notion. This means that, contrary to the beam-type device, the ITD works in two steps, i.e. ion accumulation and mass analysis. Since these are separated in time, the yield of detectable ions will become high and rather independent on the scan range. The main advantage of the ITD over the beam-type scanning approach is that the former may give nearly the same sensitivity when run in the scan as in the SIM mode. The trade-off between the extent of the mass range chosen and the test sensitivity gained, thus, becomes less crucial with the ITD than with the magnetic or quadrupole device. By the use of the ITD the analyst may, thus, scan with a high sensitivity the whole mass range that covers a substance group of interest. Given that the forensic scientist often does not know what to look for, and, therefore, needs a search method with a high sensitivity, the ITD should perhaps best meet his demand. Adrawback of the ITD is that its sensitivity is more dependent on interfering substances than the beam-type scanning mass spectrometer; the ITD sensitivity, thus, drops with increasing amounts of impurities that may be co-eluted with the analytes during the chromatographic separation. The generation of somewhat distorted mass spectra at high analyte concentrations giving rise to enhanced [M+1]+ peaks is another inherited ITD problem. The reason for this may be an overload of the ion source, an event that probably gives rise to some chemical ionization by the analyte molecules themselves.
In addition to the positive ions formed during ionization of a molecule by EI or CI, negative ions are also produced, and by changing the electric field of the mass spectrometer these can be monitored. In certain instances, i.e. when the target substances have a high affinity for electrons, negative ion monitoring can be extremely useful, mainly because of the high sensitivity that can be achieved. However, such a situation is only reached when CI, often resulting in a low fragmentation with the formation of an ionized molecule, keeps the energy of the electrons at a low level. At high electron energy, on the other hand, a number of low-mass fragments without much analytical information are formed. The approach using negative ion monitoring has been particularly fruitful for the analysis of halogenated drug substances, which have been detected at 100-1000-fold higher sensitivity than when tested by positive ion monitoring.
Data Interpretation
Apencil and paper are the classical tools the analyst uses to decode a mass spectrum for pin pointing the agent, which may be the origin of a recorded fragment pattern. By this means he tries to postulate the substance based on the features of the spectrum along with his knowledge about basic reaction mechanisms. The approach, however, entails a number of steps to reveal the elemental make-up for the main ions, the molecular ion, and the functional groups from series of ions or characteristic ions showing up in the spectrum. These efforts are done along with trial and error to predict the major fragments from often a variety of postulated structures. It is obvious that this is a clumsy and time-consuming method, which calls for great skill from the analyst, and also allows some decipherable bias. It is, thus, not suited for the everyday work.
Today the evaluation of a recorded mass spectrum is computerized. By comparing the mass spectrum revealed with reference mass spectra in a database, one looks for the agent with a spectrum that best matches that of the unknown analyte. For collating, different algorithms have been devised, and in most instances the data program produces ten candidates. Each of these is rated with a number between 0.0 for a very poor fit and 10.0 for a perfect match to the mass spectrum of the analyte. Even though the search system is based on the notion that similar mass spectra of the reference and of the unknown substance mark a chemical unity, the use of such a method requires that the operator look upon the search data with a great deal of skepticism. Acommon problem is that there is seldom a perfect match between the mass spectra of the analyte and of the reference agent in the library. This may be because the unknown substance is present only in low concentrations and some of its fragments, therefore, do not show up, or it is tainted by impurities, which make the recorded mass spectrum unsure. Also, the library may lack a corresponding reference substance or yield only abridged mass spectra to reduce the program’s search time among the often several tens of thousands of entrainees. These are situations that will make a perfect fit unlikely. Even though a computerized interpretation of mass spectra offers the operator a great help, a final verification must be made by some independent means. Preferably, a mass spectrum of the reference substance should be run under the same conditions as used for the sample test, alternatively the retention time or the retention index value of the analyte should be compared with that of the candidate compound arrived at after the mass spectrum search.
Some Forensic Applications of MS
The search for drug substances and poisons in body fluids from living persons or in organs of postmortem materials offers an important and difficult task for the chemist. In a forensic survey toxicology perhaps yields the most common use of MS, and its utility in such work is elaborated on elsewhere. It shows an example of GC/MS screening of a urine sample for drug substances with the apparatus run in the scan mode and with the library search for compounds of the main peak showing up on the mass chromato-gram. As pointed out earlier, only rather high concentrations of the ‘general unknown’ can be spotted under such test conditions.
In many instances the search of a forensic sample for trace amounts of the ‘general unknown’ requires that a MS system with higher sensitivity be used than offered by the mass spectrometer run in the scan mode. The analyst of a fire debris sample is often faced with such a need. The aim of his work is to detect and identify in a fire debris sample residues of accelerants that could have been used by an arsonist to start the fire. Since the substances screened for are volatile and, thus, can be refined by headspace extraction into a rather clean blend, the use of an ITD is seemly. Fig. 1 shows the steps involved in the MS analysis of a fire sample from a real-life event.
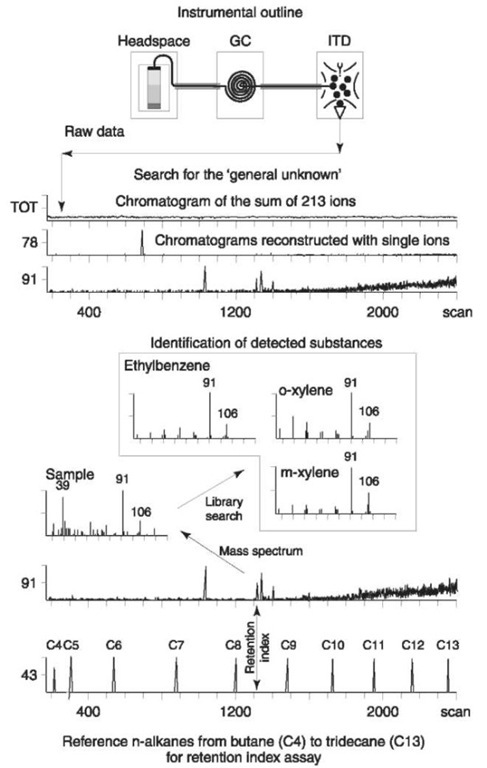
Figure 1 GC/MS search for arson accelerents in postmortem sample of actual fire victim. As shown under ‘Instrument outline’ volatile organic compounds released during the headspace extraction of the sample are chromatographed by GC, and the isolated components let directly into the ion source of the ITD. In the ‘Search for the general unknown’, the sum of all 213 ions registered is monitored as the total ion current (TOT). No peaks can be seen at this point. In the next step 213 new chromato-grams are reconstructed with each single ion, two of which are shown in the figure at m/z 78 or 91. Since a more favorable signal-to-noise ratio is obtained, a number of peaks with yet unknown identities can be seen on both reconstructed mass chromatograms. As shown under ‘Identification of detected substances’, the second peak on the mass chromatogram reconstructed with ion at m/z 91 is taken as an example. Its mass spectrum is recorded and compared with the mass spectra in a library. The mass spectrum quality of the unknown compound is, however, not good enough for picking a clear match among the three ‘hottest’ library candidates shown in the figure. To make a final identification the mass spectrum data are supplemented with retention index values indicating that the unknown substance is ethylbenzene.
