Introduction and Background
Capillary electrophoresis (CE) (also known as high-performance capillary electrophoresis (HPCE)) was devised about 20 years ago and, essentially, can be regarded as the instrumental optimization in capillaries of traditional electrophoretic techniques. This somewhat elaborate process of ‘evolution’ took about a decade to accomplish and by the early 1990s the technique was virtually ready for significant practical applications. Since then, CE has had extensive coverage in a variety of scientific domains, including chemistry, biochemistry, pharmacology, toxicology, and biomedicine. A survey in December 1998 discovered more than 5000 internet web pages matching the key words capillary electrophoresis, an increase of 3000 since the previous year.
One of the reasons leading to this clear success is that CE is indeed a very versatile technique. It enables separation of a variety of molecules – from small inorganic ions to huge biopolymers – with much the same equipment, although using separation modes based onto completely different physicochemical mechanisms. Another is that highly sophisticated, user friendly, and very effective CE instruments have become commercially available at affordable costs.
Typically, CE separations are highly efficient (up to millions of theoretical plates), sensitive (in terms of mass and concentration), fast (usually < 30 min), simple (no derivatization needed), and require extremely small amounts ofsolvents (fewtens ofml per day of running buffer) and other consumables (e.g. capillaries).
Recently, there has been great interest in CE in the world of forensic science with the publication of review articles and journals’ special issues on this subject.
The purpose of this article is to introduce some basic concepts of CE for the general forensic science audience and to provide a comprehensive overview on the most outstanding applications of this technique to a variety of forensic disciplines, includes forensic toxicology, forensic biology/biochemistry (DNA profiling, analysis of proteins and other biological compounds of forensic interest), explosive and gunshot residue analysis, and ink analysis.
Instrumentation
A CE instrument, also called a capillary electropherograph, is so simple, that ‘home made’ instrumentation can be developed for specialized applications, research, or tutorial purposes. However, the best analytical performances, in terms of reproducibility, accuracy and automation can only be achieved with commercial instrumentation.
Briefly, a capillary electropherograph (Fig. 1) consists of an injection system, a capillary, a high voltage source (10-30 kV), electrodes and electrode jars, and a detector.
In CE, the separation occurs in a capillary and this is also the compartment where injection and detection takes place. Ideally a capillary should be chemically and physically resistant, precisely machined with narrow internal diameters (typically i.d. 20-100 um; length of 20-100 cm), not prone to adsorb solutes, transparent to UV-visible radiation, and should have a high thermal conductivity and low cost. Fused silica capillaries with an external protective polyimide coating (similar to those used in capillary gas chromatography) meet almost all these requirements and are consequently a standard choice in CE. Capillaries can be internally ‘uncoated’, having the inner silica surface in direct contact with buffers and solutes, but, under some experimental conditions, these may adsorb analytes. Alternatively, the capillaries can be internally coated with a thin layer of polymers (adsorbed or chemically bound) shielding the silica surface from interactions with solutes. Capillaries can be also filled with gels, mimicking slab gel electro-phoresis, or with particles carrying a stationary phase, reproducing a chromatographic system.
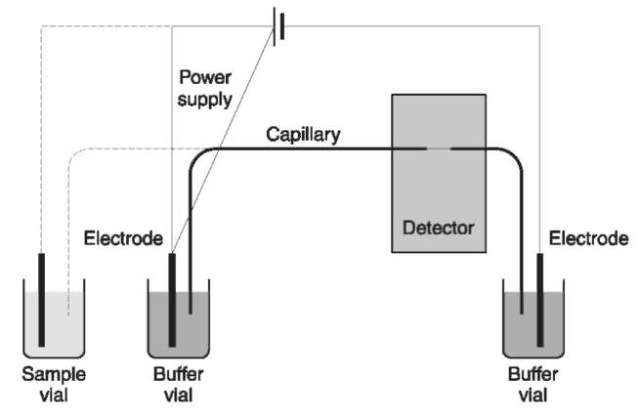
Figure 1 Schematic representation of a capillary electropherograph; interrupted lines indicate the different capillary and electrode locations during electrokinetic injection.
At the capillary wall, a crucial phenomenon, such as the so-called electroosmotic flow (EOF), takes place. It is generated because the inner wall of the fused silica capillary exposes a great number ofsilanol groups to the buffer, which are negatively ionized (as SiO-) at pH values higher than about 4 and which interact electrostatically with the ions in solution. Thus, cations, in the case of silica capillaries, are attracted and concentrated at the interface with the capillary wall and, when a potential difference is established between the ends of the capillary, migrate towards the cathode (negative electrode) drawing water with them by osmosis. This generates a flow of liquid inside the capillary which is named EOF. A peculiarity of this liquid flow is that, being generated at the capillary wall, it has a flat profile (piston-like). This limits the band-broadening that occurs during CE separations, which is a typical drawback of capillary liquid chromatography, where the flow of the mobile phase is generated by pressure. Because of its nature, EOF is highly affected by pH, ionic strength and composition of the buffer. To abolish (or reverse) the EOF the capillary wall can be coated with ‘modifiers’, which can be physically adsorbed, chemically bound or simply added to the running buffer (dynamic coating). The most common coatings are polyacrylamide, cellulose, polyvinyl alcohol (PVA), amino acids, amines, surfactants, aryl pentafluoro compounds, poly(vinylpyrrolidinone) or polyethyle-neimine etc.; polymers well known as liquid chroma-tographic (C2,Cg,C1g) or gas chromatographic stationary phases (polyethylene glycol (PEG), phenyl-methyl silicone) can be used for this purpose.
Due to the minimal total volume (ul) of the capillary the injection volume must not exceed few tens of nl (1-2% of the total volume), if a high separation efficiency is to be maintained in the system. The injection of these minute amounts of liquids poses problems of accuracy and precision, but modern instruments can assure reproducibility better than 2%. With the use of internal standards an even better precision can easily be achieved.
Modern instrumentation uses two injection principles: hydrodynamic or electrokinetic. In the first case, a pressure difference is generated between the two ends of the capillary, while the injection end is dipped in the specimen vial. Alternatively, a voltage difference is established between the sample’s and the opposite vial, while samples are being injected. Although hydrodynamic injection (pressure driven) is nonselective (i.e. what is injected is representative of the sample composition), electrokinetic injection (potential driven) is selective. This is because the sample components enter the capillary according to their electrophoretic mobility, the mobility and concentration of total ions in the sample and the EOF (see in the next section). Electrokinetic injection allows field-amplified sample stacking, i.e. a highly efficient method for increasing analytical sensitivity (in terms of concentration), to be accomplished.
The high-voltage power supplies used for CE are generally able to give voltages up to 20-30 kV and currents up to 200-300 uA. Separations are generally carried out under constant potential, but voltage gradients or steps; and constant current separation is sometimes used. Because EOF in fused silica capillaries is usually directed towards the cathode, the common polarity in a capillary electropherograph is with the anode at the injection end of the capillary and the cathode at the opposite end, close to the detector; however, under specific analytical conditions, the polarity is reversed.
CE detection, most often with UV-visible radiation, is carried out in-capillary, in order to avoid any possible postseparation added volumes. This can cause unacceptable band spreading (Fig. 1). Indeed, the wall of fused silica capillaries, after removing the polyimide coating, is highly transparent. Thus, pico-gram amounts of analytes can easily be detected even by UV absorption, but, due to the limitations in the sample volume (nl) which can be injected, the concentration sensitivity with UV detectors is limited to 10-<5M. However, this limit in sensitivity can be overcome by using other detection techniques, e.g. laser-induced fluorescence (sensitivity up to 10-12M) or electrochemical detection (sensitivity up to 10-8M) and/or by adopting high efficiency sample stacking methods, which can assure improvements in sensitivity of 2-3 orders of magnitude.
In current instrumentation, detection is most often based on UV(-visible) absorption; implementation of diode-array or fast-scanning UV detectors allows on-line recording of the UV spectra of the peaks, improving the information content of the CE analysis. Fluorescence (mostly, laser induced), electrochemical detection (conductimetric) and mass spectrometric (generally with electrospray ionization) detection modes have recently become commercially available. Other detection modes (including amperometric detection, thermooptical detection, chemilumines-cence etc.) are still under development or need special instrumental arrangements.
A detection mode which has gained popularity in CE is the so-called ‘indirect detection’, which allows the determination of ionic molecules, such as small inorganic and organic ions, that are not directly detectable by the used CE detectors (e.g. do not absorb UV, are not fluorescent). Indirect detection is based on the addition to the running buffer of a ionic additive which is detectable at trace levels by the detector. This ionic compound, having the same charge and similar mobility to the analyte(s) of interest, is displaced from the zones where the analyte(s) migrate to preserve electroneutrality, and this displacement gives rise to ‘reversed peaks’, the area of which is proportional to the concentration of the given analyte(s). Drawbacks of this detection mode are sensitivity, which is usually lower than with the corresponding ‘direct’ mode, and the narrower range of linearity.
Separation Techniques
One of the most interesting characteristics of CE is that with the same instrumental hardware separations based on different physicochemical principles can be carried out. The most common CE separation techniques include: capillary zone electrophoresis (CZE), micellar electrokinetic capillary chromatography (MECC or MEKC), capillary electrochromato-graphy (CEC), capillary isoelectric focusing (CIEF),capillary gel electrophoresis (CGE), capillary isota-chophoresis (CITP) and capillary electrophoretic immunoassay (CEIA), which can be accomplished by simply changing the composition of the running buffer and the type of capillary.
Capillary zone electrophoresis (CZE)
CZE is a free-solution high-voltage electrophoresis in electrolyte-filled capillaries. Separation is based on the different electrophoretic mobility of analytes (u.e), which under an electric field (E) migrate as sharp zones at different velocity (v = u.e E).
ue is described by the following equation:
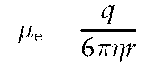
where q = ion charge, r = ion radius, r\ = solution viscosity.
For weak acids and bases, the charge and effective mobility (ueff) is dependent on the pK values of the different ionizable groups and on the pH of the running buffer.
In particular, for a weak acid
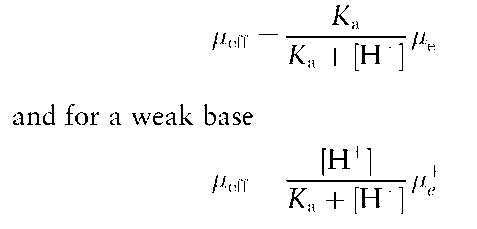
Besides electrophoretic migration, a fundamental factor in CZE is electroendoosmosis. EOF produces an electrically driven flow of liquid, which in fused silica capillaries is directed from the anode to the cathode.
The linear velocity of the electroosmotic flow is described by the following equation:
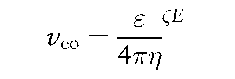
where s is dielectric constant, L is zeta potential, r\ is solution viscosity and E is electric field.
The usual arrangement of a capillary electropher-ograph is such that injection is at the anodic end and detection is close to the cathodic end of the capillary. Considering also that EOF is generally oriented towards the cathode and that it is greater than the electrophoretic velocity of most analytes, it follows that cations, neutral species and anions will reach the detector in this sequence.
In fact, the migration velocity of cations and anions will result from the algebraic sum of their electro-phoretic velocity and that of EOF. All neutral species migrate at the same velocity as the EOF and consequently can not be resolved.
In the presence of the EOF, the migration velocity of the analytes follows the equation:
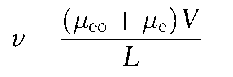
where u.eo and u.e are mobilities of EOF and of the analyte, respectively, V is potential and L is capillary length.
In CZE, the most important experimental factors controlling the separation are applied voltage, capillary length, buffer pH and composition, additives and capillary wall modifications.
The ideal CZE background buffer should have good buffering capacity at the chosen pH, low conductivity (to let high potentials act without unbearable heat production), buffer ion mobility matched to that of the analyte (to avoid peak distortion), and negligible interference with the used detection technique (e.g. low background UV absorbance at the adopted UV wavelength).
In practise, high buffer concentrations have been shown to reduce the wall-to-analyte interactions and, for a better buffering capacity, relatively high molar-ity background buffers are preferred in CZE, compatible with the resulting currents.
For most purposes, phosphate, borate, citrate and phosphate-borate buffers can be used. Zwitterionic buffers (Tris, 3-[(cholamidopropyl)dimethylammo-nio]-1-propane-sulfonate (CHAPS) etc.) are sometimes necessary in order to work at high concentrations without excessive currents (and consequently too high temperatures in the capillary generating loss of efficiency).
Buffer additives can greatly affect selectivity and resolution, introducing in the electrophoretic separation process new interactions and consequently additional selectivity. The most important additives in CE include: organic solvents (e.g. methanol, acetonitrile, isopropanol, tetrahydrofuran), anionic (e.g. sodium dodecyl sulfate (SDS)), cationic (e.g. cetyltrimethyl-ammonium bromide (CTAB)) or neutral surfactants, organic amines, metal ions, urea, linear polymers (PEG, polyacrylamide, alkylated cellulose), complex-ing agents. Finally, if the nature of interactions between additives and analytes are stereoselective (with for example bile salts, cyclodextrins), a chiral selectivity can be achieved by the CE separation.
Micellar electrokinetic capillary chromatography (MECC)
As discussed above, in CZE, the charged molecules undergo electrophoretic separation, whereas all the neutral substances are pushed by the EOF to move at the same velocity towards the detector and, consequently, are not separated.
Around the middle of the 1980s, micellar electrokinetic capillary chromatography (MECC) (known also as MECK) was developed to resolve uncharged compounds by Prof. S. Terabe.
In this separation technique, an ionic surfactant forming micelles is added to the buffer and interacts with the solutes according to partitioning mechanisms. In this quasi-chromatographic system, EOF acts as the chromatographic ‘mobile phase’ pushing ana-lytes (and micelles) towards the detector. The micelles represent the ‘stationary phase’ (better called ‘pseudo-stationary’), and, because of their charge, tend to migrate backwards and selectively retard the analytes they interact with.
In a very common MECC system, the micellar phase is composed of the anionic surfactant SDS and the resulting anionic micelles are electrostatically driven towards the anode. However, because of the prevalence of EOF (running buffer is basic and generates a strong EOF directed towards the cathode), moving in the opposite direction, the resulting micelle migration is in the direction of the detector (close to the cathode). Consequently, the micelles selectively affect the ‘mobility’ of nonionic solutes (which otherwise equals that of EOF) they interact with. A selectivity based on partitioning of solutes between buffer and the lipophilic core of the micelles is thus introduced.
For ionizable/ionized solutes, separation mechanisms include electrostatic interactions between the ionic forms of the analytes and the charged surface of the micelles (ion-exchange like).
Selectivity changes depend on the nature of the micelles. Besides SDS and other anionic surfactants, other surfactants commonly used in MECC are hydrophobic-chain quaternary ammonium salts. The latter invert the EOF and consequently separations require reversed polarity.
Other factors crucial for the fine tuning of selectivity, such as organic solvents (methanol, isopropanol, acetonitrile, tetrahydrofuran), may be added to the separation buffer, in a similar way to reversed-phase chromatography. Also, urea (2-6 M) is known to increase the solubility in water of hydrophobic molecules and, in MECC, the separation of very lipophilic compounds is reported to be improved by highly concentrated urea.
Microemulsion capillary electrophoresis (MCE), using oil-in-buffer microemulsions instead of surfactant micelles, exerts a separation mechanism similar to MECC, but, so far, it has found limited application and is still mostly at an experimental level.
Capillary electrochromatography (CEC)
In CEC, a chromatographic stationary phase is contained in the capillary (packed or wall-immobilized) interacting with the solutes according to the usual chromatographic separation mechanisms. The mobile CEC phase is driven through the capillary by electro-osmosis, not by pressure, as occurs in chromatogra-phy. This allows high resolving power and efficiency.
CEC is still at an early stage of development but looks particularly promising for separation of neutral hydrophobic molecules and in view of its coupling with MS.
Capillary isotachophoresis (CITP)
CITP is the capillary counterpart of classical isota-chophoresis. In CITP, the ionic compounds migrate in discrete zones, at the same velocity, between a leading and a terminating electrolyte, have the highest and the lowest mobility of all analytes, respectively. CITP principles may be applied not only for separation, but also for sample pretreatment, before CZE, achieving concentration factors in excess of 100 times.
Capillary gel electrophoresis (CGE)
Gel electrophoresis is still the standard method for the separation and characterization of proteins (SDS-polyacrylamide gel electrophoresis (PAGE)), DNA fragment mapping and DNA sequencing. CGE is its instrumental evolution.
CGE capillaries may contain chemically cross-linked gels or noncrosslinked linear polymer matrices. Although they have higher efficiency and resolving power, capillaries filled with crosslinked gels are very delicate and prone to clogging. An alternative is represented by noncrosslinked sieving gels, generated by the physical entanglement of linear polymers (alky-lated celluloses or linear polyacrylamide) dissolved at suitable concentrations in the running buffer. In effect, the noncrosslinked polymers exert a sieving mechanism similar to traditional crosslinked gels, but remain fluid and can be replaced by refilling the capillary by pressure application.
Capillary isoelectric focusing (CIEF)
CIEF is the replication in the capillary of slab gel isoelectric focusing, a popular separation mode in protein separation. In the classical isoelectric focusing, pH gradients are formed by letting a myriad of amphoteric compounds (ampholytes, the industrial product of the chemical breakdown of acrylic acid) arrange themselves side-by-side according to their isoelectric point (pi at which the net surface charge is zero), as long as a stable (constant voltage) electric field is established. At equilibrium, proteins are placed in the gel and are conveyed to the pH point corresponding to their pI (at isoelectric point, charge and mobility are null and there is no further migration). The same principles apply to CIEF, with the notable differences that separation is carried out in free solution, and that, after focusing, the various isoelectric bands have to be mobilized to the detector by hydrodynamic or electroosmotic methods.
Chiral separations
The separation of chiral compounds is gaining increasing attention in pharmacological/pharmaceutical science as well as in forensic toxicology. In reality, CE most often allows chiral separation to occur directly in the liquid phase, without the need of derivatization or of chiral stationary phases. The chiral selectors used in CE include cyclodextrins (CDs), Cu(II)-L-histidine, Cu(II)-aspartame, bile salts, crown ethers, proteins (bovine serum albumin, a1-acid glycoprotein). Chiral resolution results from stereospecific interactions of the chiral selector with the two enantiomers giving rise to a difference in mobility between the two entities.
Capillary electrophoretic immunoassays (CEIA)
Coupling competitive immunoassays with CE-driven bound/free fraction separation has recently gained considerable attention, particularly whenever fluorescent tracers are used. Both CZE or MECC can be used to separate the bound from the free fraction of the tracer in a competitive immunoassay, allowing indirect but highly sensitive quantitation of several analytes in a variety of biological samples. Additionally, since different tracers can be easily spotted in one electropherogram, multicomponent immunoassays can be simultaneously carried out in the same experiment. Many commercial immunoassay reagents can be adapted to CEIA.
Applications
Illicit drug analysis and forensic toxicology
Since its start, CE has been applied to the analysis of drugs and pharmaceuticals and in pharmaceutical research and development. Although much later, CE has also been proposed as a tool for illicit drug analysis in both clandestine preparations and biological samples.
The first application of MECC to the analysis of illicit drug substances achieved the separation of a broad spectrum of compounds of forensic interest, including psilocybin, morphine, pheno-barbital, psilocin, codeine, methaqualone, lysergic acid diethylamide (LSD), heroin, amphetamine, chlordiazepoxide, cocaine, methamphetamine, lora-zepam, diazepam, fentanyl, phencyclidine, cannabi-diol and tetrahydrocannabinol. The protocol implied the use of 50 um i.d. bare fused silica capillaries (length: 25-100 cm) using a buffer consisting of 8.5 mM phosphate, 8.5 mM borate, pH 8.5, 85 mM SDS and 15% acetonitrile; the applied voltage was 25-30 kV and detection was by UV absorption at 210 nm. Fluorescence detection (excitation wavelength 257 nm, emission 400 nm) was also successfully tested for fluorescent analytes (containing phenanthrene rings). Highly efficient separations of heroin acidic and neutral impurities, degradation products and adulterants have been obtained from seized heroin samples, and from street cocaine samples, the simultaneous resolution of benzoylecgonine cocaine, as well as cis- and £ra«s-cinnamoylcocaine have been obtained.
In this first application, the analytical precision was characterized by a relative standard deviation (RSD) of about 0.5% for migration times and 4-8% for peak areas and peak heights (although peaks with migration times >40min had worse precision). This phenomenon was ascribed to a rapid ‘aging’ of the buffer due to evaporation of organic solvent and water electrolysis. Frequent changes of the buffer and the use of electrolyte solutions with high buffering capacity was recommended to improve reproducibil-ity. Additionally, since MECC and reversed-phase HPLC yielded uncorrelated elution patterns, they could be used as mutual countercheck procedures.
MECC, under similar conditions, has been used in combination with a fast-scanning UV detector for the analysis of illicit preparations of heroin and cocaine resulting in the identification of the individual peaks on the basis of both migration times and UV spectra.
Several other authors have confirmed the suitability of MECC with either anionic or cationic micelles to analyze clandestine preparations of illicit drugs.
Comparison of MECC with HPLC and GC showed good quantitative correlation between the different techniques. Precision was slightly worse than with HPLC, but the resolution of complex samples was better with CE. Also, CE proved highly reliable in interlaboratory proficiency tests for illicit heroin and cocaine analysis.
In drug screening, CZE proved to be a realistic alternative to MECC when using plain 50 mM phosphate buffer, pH 2.35, in a 75 um i.d. (60 cm long) bare fused silica capillary. Detection was by UV absorption at 214 nm. In only 11 min, the separation of 17 basic drugs of forensic interest including methapyrilene, brompheniramine, amphetamine, methamphetamine, procaine, tetrahy-drozoline, phenmetrazine, butacaine, medazepam,lidocaine, codeine, acepromazine, meclizine, diaze-pam, doxapram, benzocaine and methaqualone was achieved. Drugs with lower pKa values and consequently less positive charge, showed higher migration times, but other factors (such as molecular size, tendency to interact with the column and ability to form doubly charged species) affected electrophoretic mobility, allowing the resolution of molecules with the same charge-to-mass ratio. RSDs of migration times were <1%, whereas peak-area RSDs ranged from 1.5 to 4.3% (worse reproducibility was found for analytes with pKa values close to the pH of the background buffer and, consequently, with very slow migration). CZE may have advantages over MECC for drug screening and, particularly, simple background electrolyte preparation and shorter analysis times. The main limitation is the inability to analyze acidic, neutral and basic drugs simultaneously, as is possible with MECC.
The CZE mobility of as many as 550 basic (analyzed in 100 mM phosphate, pH 2.38) and 100 acidic drugs (analyzed at pH 8.50) has been determined for the rapid screening of blood samples.
The complementary nature of CZE and MECC for the identification of 17 illicit drugs and related compounds has been clearly demonstrated in a study in which MECC with SDS at pH 9.2 gave highly non-correlated separation with CZE at pH 2.35.
Chiral discrimination is of primary importance in the analysis of amphetamine substances for investigations on the synthetic methodologies and CE is the most effective analytical tool for this purpose. In effect, it offers high efficiency chiral resolution, high speed and flexibility, low costs, and no need of sample derivatization. After preliminary attempts by using derivatization with a chiral reagent (2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl isothiocyanate) to form diastereo-isomers, followed by MECC, direct chiral analysis has been introduced by using native or deri-vatized p-CDs and this is now a standard methodology for the separation of enantiomers of amphetamine/methamphetamine, including ring substituted analogues.
CE was introduced in the field of forensic and clinical toxicology with a MECC method based on a phosphate-borate buffer, pH 9.1, with 75 mM SDS for the qualitative determination in urine of drugs of abuse (and their metabolites) including benzoylecgo-nine, morphine, heroin, 6-monoacetylmorphine (MAM), methamphetamine, codeine, amphetamine, cocaine, methadone, methaqualone and some major benzodiazepines.
Sample extraction was carried out by using commercially available ‘double mechanism’ (cation exchange and reversed-phase) solid-phase extraction cartridges widely adopted in GC and HPLC. The extract from 5 ml of urine was dried and the residue redissolved with 100 ul of running buffer and injected. Under these conditions, a detection limit of 100ngml_1 was easily achieved, meeting the required sensitivity for confirmation of current immu-noassays. Peak identification was also achieved by comparison of the on-line recorded UV spectra of the peaks with computer-stored models of known compounds.
Similar MECC approaches, based on separations in phosphate-borate/SDS buffers and multiwavelength UV detection, have been proposed for the determination of many drugs of abuse in biological fluids, in some instances without any sample pretreatment. However, CZE also proved suitable for this purpose.
Table 1 summarizes some paradigmatic methods for the determination of a wide range of abused substances.
Although drugs administed at high doses, such as barbiturates, may be determined directly by CE, the relatively poor sensitivity in terms of concentration of this technique most often necessitates that the sample is extracted and concentrated before injection. A stepwise solid-phase extraction of the most common drugs of abuse from human urine, preliminary to MECC, used commercial cartridges exhibiting hydrophobic and ion-exchange interactions and was reported to give ‘clean’ electropherograms even with 50-fold concentrated urine. It has also been observed that MECC with SDS in plain aqueous buffers failed to resolve some analytes (e.g. amphetamine, metham-phetamine, methadone and benzodiazepines) and that it could be obtained by adding low percentages of acetonitrile (5-10%).
Table 1 Selected CE methods for analysis of drugs of forensic interest in biological fluids
| Analyte | Sample/sample | Method |
| preparation | ||
| Benzoylecgonine, morphine, heroin, 6-MAM, codeine, | Urine/SPE | MECC: uncoated fused silica capillary, |
| methamphetamine, amphetamine, cocaine, | phosphate-borate buffer pH 9.1 with | |
| methadone, methaqualone, benzodiazepines | 75 mM SDS; detection: UV | |
| Barbital, allobarbital, phenobarbital, butalbital, | Urine/SPE | MECC: uncoated fused silica capillary, |
| thiopental, amobarbital, pentobarbital | Serum/direct | phosphate-borate buffer pH 7.8 with 50 mM |
| SDS; detection: UV | ||
| 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid | Urine/SPE, | MECC: uncoated fused silica capillary, |
| (THC metabolite) | hydrolysis | phosphate-borate buffer pH 9.1 with 75 mM |
| SDS; detection: UV | ||
| Morphine-3-glucuronide | Urine/SPE | MECC: uncoated fused silica capillary, |
| phosphate-borate buffer pH 9.8 with 75 mM | ||
| SDS; detection: UV | ||
| CZE: uncoated fused silica capillary, | ||
| phosphate-borate buffer pH 9.8: | ||
| Ephedrine, norephedrine | Urine/direct | CZE: uncoated fused silica capillary, 50 mM |
| phosphate buffer pH 9.5, 1% acetonitrile; | ||
| detection: UV | ||
| Acebutolol, alprenolol, atenolol, labetalol, metoprolol, | Urine/direct or | MECC: uncoated fused silica capillary, 80 mM |
| nadolol, timolol oxprenolol, pindolol, propranolol, | SPE | phosphate buffer pH 7 with 10mM CTAB; |
| detection: UV | ||
| Methapyrilene, brompheniramine, codeine, lidocaine, | Urine/LLE | CZE: uncoated fused silica capillary, 50 mM |
| amphetamine, methamphetamine, meclizine, | Serum/LLE | phosphate buffer pH 2.35; detection: UV |
| procaine, tetrahydrozoline, phenmetrazine, | ||
| butacaine, medazepam, acepromazine, diazepam, | ||
| doxapram, benzocaine, methaqualone | ||
| Flunitrazepam, diazepam, midazolam, clonazepam, | Urine/SPE | MECC: uncoated fused silica capillary, |
| bromazepam, temazepam, oxazepam, lorazepam | phosphate-borate buffer pH 9.3 with 75 mM | |
| SDS; detection: UV | ||
| Methadone, EDDP(urinary metabolite) | Urine/SPE | CZE: uncoated fused silica capillary, 50 mM |
| borate buffer pH 8.9; detection: UV | ||
| Clenbuterol | Urine/LLE | CZE: uncoated fused silica capillary, 25 mM |
| citrate buffer pH 5; detection: UV | ||
| 26 tricyclic drugs, including phenotiazine, imipramine, | Urine/LLE | MECC: uncoated fused silica capillary, 40 mM |
| promazine, amitriptyline, clozapine, amiodarone, | borate buffer pH 9.5 with 10 mM Na | |
| prochlorperazine, chlorpromazine, etc. | taurodeoxycholate; detection UV | |
| Pholcodine, 6-MAM, morphine, heroin, codeine, | Urine/SPE | CZE: uncoated fused silica capillary, 100 mM |
| dihydrocodeine | phosphate buffer pH 6.0; detection: UV | |
| LSD, nor-LSD, iso-LSD, iso-nor-LSD | Blood/LLE | CZE: uncoated fused silica capillary, 250 mM |
| citrate/acetate buffer pH 4.0-methanol, | ||
| 30:70; detection: LIF | ||
| Amphetamine, methamphetamine and related | Urine/LLE-SPE | CZE: uncoated fused silica capillary, 50 mM |
| compounds | Derivatization | acetate buffer pH 3.0; MECC: 50 mM borate |
| buffer pH 9.3 with 15 mM SDS; detection | ||
| UV-LIF |
Hair analysis is an innovative field of forensic toxicology, shedding light on former, chronic exposure to illicit drugs. The usual analytical strategy is based on hair extraction, extract purification, immu-nological drug screening and chromatographic (GC, GC-MS, HPLC) confirmation. To this aim, CE offers advantages in terms of peculiar separation mechanism and minimal need of hair sample.
By using a CZE method based on an uncoated fused silica capillary, a background buffer composed of 50 mM borate, pH 9.2 and UV detection, the simultaneous detection of cocaine (238 nm) and morphine (214 nm) has been accomplished. The use of sample stacking techniques allowed a > 100-fold increase in sensitivity (Fig. 2), with the possibility of recording UV spectra of the drugs in hair.
Due to the fundamental role of mass-spectrometry (MS) in modern analytical toxicology, great attention is at present being paid to the CE-MS coupling. The development of on-line CE-MS started in the late 1980s and more recently commercial instruments with electrospray interfaces have appeared (CE-ESI-MS). CE-MS has been introduced in the pharmaceutical field and to some extent in forensic toxicology.
Analysis of gunshot residues and explosive constituents
The analysis of gunshot and explosive residues, for intelligence or courtroom purposes, is a fundamental area of forensic science.
Both inorganic and organic constituents are of crucial interest and are currently analyzed by the most sophisticated techniques, including scanning electron microscopy neutron activation analysis, mass spectrometry, X-ray and infrared techniques, and all types of chromatography. CE can complement more established methods, especially in the analysis of organic and inorganic compounds.
In this field, the first contributions date back to the early 1990s, when MECC was used for the separation and determination of the major organic constituents in reloading powders, explosive materials and in gunshot residues. The separation method used uncoated fused silica capillaries and a buffer composed of 2.5 mM borate, 25 mM SDS; detection wavelength was 250 nm (200 nm for nitroglycerin). As many as 11 components of gunshot residues (including nitroguanidine, nitroglycerin, 2,4-dinitrotoluene (DNT), 2,6-DNT, 3,4-DNT, 2,3-DNT, diphenyla-mine (DPA). N-nitrosoDPA, 2-nitroDPA, ethylcen-tralite and dibutyl phtalate) and 15 high-explosive constituents (including nitroguanidine, ethylene gly-col dinitrate, diethylene glycol dinitrate, 1,3,5-trini-tro-1,3,5-triazacyclohexane (RDX), nitroglycerin, 2,4,6-trinitrotoluene (TNT), pentaerythritol tetrani-trate, picric acid) were successfully resolved in 10min. In addition, since several analytes have characteristic UV spectra, multiwavelength absorbance analysis helped their identification. Application to forensic cases included the investigation of spent ammunition casings, reloading powders and plastic explosives (Semtex, C4, Detasheet, Tovex).
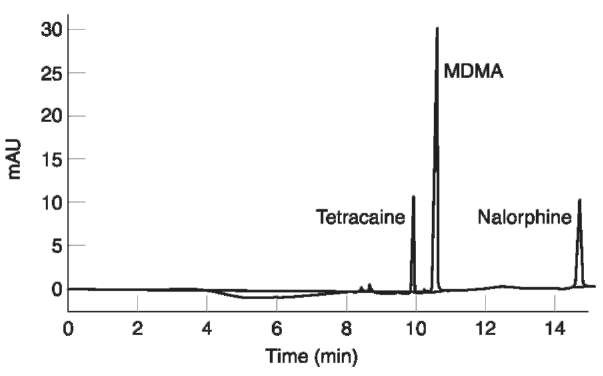
Figure 2 Electropherogram of an extract from a sample of hair from a user of ‘ecstasy’ containing 3,4-methylenedioxymetham-phetamine (MDMA) at a concentration of 4.0ng mg-1. Tetracaine and nalorphine were added as internal standards. Injection was by electromigration under field amplified sample stacking conditions. Separation was by CZE using 100 mM phosphate pH 2.5 as the running buffer. Detection was by UV absorbance at 200 nm. (Reprinted with permission from Tagliaro F, Manetto G, Crivellente F, Scarcerlla D and Marigo M (1998) Hair analysis for abused drugs by capillary zone electrophoresis with field-amplified sample stacking. Forensic Science International 92: 201-211.
Specific sample collection procedures for the CE analysis of gunshot residues have been developed, based on the use of masking adhesive tape (1 inch square sections). The film lifts were examined with a binocular stereoscope and the gunshot residue particles collected with tweezers and analyzed by MECC. This adhesive film collection was found to be clearly superior to the traditional swabbing. By the combined use of improved collection and microsample analysis, determination was possible of individual particles collected at the skin surface. Firing range experiments with subjects trying different weapons demonstrated that characteristic gunshot residue constituents could be recovered by adhesive film lifts from both the weapons and the hands (Fig. 3).
In the case of home-made explosives (the so called low-explosives), anions and cations left behind from the blast represent useful pieces of evidence to determine the type and source of the explosive mixture used. Moreover, traces of the explosive mixture can be looked for in the environment where the device was assembled and/or on the perpetrator.
Although ion chromatography is the traditional tool for this purpose, CE can be considered a useful complementary method. To analyze postblast anions, CZE was used in borate buffer (2 mM borate, 40 mM boric acid) with 1 mM diethylenetriamine as EOF modifier at a final pH of 7.8 and a dichromate chromophore (1.8 mM); detection was indirect by UV absorbance at 280 nm. By using this method, chloride, nitrite, nitrate, sulfate, sulfide, chlorate, carbonate, hydrogencarbonate, cyanate, thiocyanate and perchlorate could be determined in a single run. A comparison of CZE with ion chromatography in real cases showed major differences between the two separation patterns, allowing mutual confirmation of the results. The reproducibility of the migration times in CZE using the same capillary was characterized by day-to-day RSDbetter than 1%, and could be further improved by adding bromide as a marker. Practical tests were carried out on pipe-bomb fragments experimentally prepared with different explosive mixtures (potassium chlorate-vaseline; black powder; smokeless powder; a mixture of black and smokeless powder) and detonated. Fragments from bombs were simply extracted with water, filtered and analyzed.
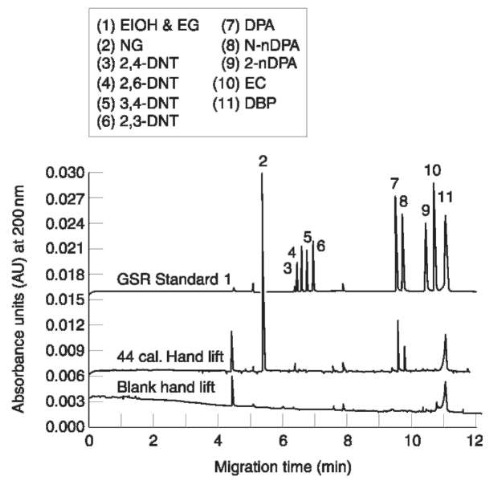
Figure 3 Electropherograms from MECC analyses of (top) a solution of gunshot residue standards, (middle) an extract of a single gunshot residue particle on a film lift from a hand which fired a 44 caliber revolver and (bottom) an extract from a film lift blank. Buffer: 25 mM sodium dodecyl sulfate, 2.5 mM borate, pH 8.5; voltage: 25 kV. (Reprinted with permission from Northrop and MacCrehan (1992) J. Liquid Chromatography 15(6):1061-1063.)
A similar CZE approach has also been used to determine inorganic anions and cations, such as nitrate, chlorate and ammonium as postblast residues of chlorate or emulsion explosives.
Analysis of pen inks
In forensic laboratories, TLC, column chromatogra-phy and slab gel electrophoresis have been used to investigate ink composition, but, again, CE has been applied with very encouraging results.
The water-soluble components of black inks from 26 marking pen specimens were analyzed by CZE using 15 mM borate buffer, pH 8.8, uncoated capillary and UV detection at 214 nm. CZE at basic pH (100 mM borate, pH 8.0, with 20% methanol) has also been used with diode array UV and LIF detection, to reliably classify almost all the components of 17 blue and black ink samples from different manufacturers. However, only initial results have been reported from dried paper-extracted inks.
Separation of biopolymers
Proteins CE has been widely used for peptide and protein analysis, but so far very little in the forensic field.
A subject of crucial interest in forensic (and clinical) medicine is the objective diagnosis of chronic alcohol abuse.
Although acute ethanol intoxication can be diagnosed by measuring blood concentrations, objective diagnosis of chronic/subchronic excessive intake is still an open problem. To this aim, several methods have been proposed including ethanol metabolites and congeners, enzymes, acetaldehyde adducts, high density lipoprotein cholesterol, 5-hydroxytryptophol and 5-hydroxytryptophol-3-acetic acid, dolichols etc. Among these, carbohydrate-deficient transferrin (CDT) is considered the most reliable objective piece of evidence of continuous excessive alcohol consumption.
The basis of CDT is a microheterogeneity of serum transferrin, the major iron transporting glycoprotein, which may contain from 0-8 sialic acid residues. The major human transferrin isoform (>90%) contains four sialic acid residues, but, as a result of alcohol abuse, less glycosylated isoforms (disialo- and asialotransferrin) are reported to increase and are collectively named CDT. The exact mechanism of this phenomenon has not yet been clarified, but, on the basis of a large body of literature, it is believed that an ethanol intake > 50-80 g day-1 for one to two weeks leads to an abnormal increase of CDT, which has a half-life of about 15 days. Diagnostic specificity, a crucial parameter for CDT application in the forensic environment, is reported to be good-to-excellent (90100%) with a sensitivity of 50-90%.
The current determination of CDT is based on immunoassays coupled to sample pretreatment using ion-exchange cartridges. More sophisticated methods of analysis include isoelectric focusing combined with immunofixation, zone immunoelectro-phoresis or Western blotting, anion-exchange chromatography or chromatofocusing followed by immunoassays and HPLC with UV detection.
CE analysis of CDT has been optimized and validated in clinical cases and its application to a forensic context is today a realistic option.
A simple CZE method using 100 mM borate buffer pH 8.3 and uncoated capillaries (20 um i.d.) with UV detection at 200 nm wavelength proved able to separate and determine quantitatively disialo- and trisia-lotransferrin isoforms (i.e. the glycoforms of major clinical relevance) in human serum within a reasonable time (20 min) and without the need for complex sample pretreatment (limited just to iron saturation and 1/10 dilution in water).
Other proteins of forensic interest which are susceptible of CE analysis include human protein variants used as phenotypic markers for identification, hemoglobins and saliva and semen proteins.
DNA fingerprinting The crucial importance of DNA analysis in modern forensic science (personal identification, paternity testing) need not be further emphasized here. However, CE is increasingly being applied in the field, particularly since the introduction of fully automated multicapillary instrumentation dedicated to nucleic acids analysis (polymerase chain reaction (PCR) amplicons and DNA sequencing).
The analysis of DNA polymorphisms is today largely focused on length polymorphisms and sequence variation. Length polymorphisms are by far the most important class of genetic variability used to identify humans. Electrophoresis on agarose or polyacrylamide slab gels is the traditional procedure for determining individual genotypes. Generally, short (<1000 base pairs long: amplified fragment length polymorphisms, AmpFLPs) or very short (down to 200 base pairs; short tandem repeats, STRs) products of PCR are targeted, differing by as little as 1% of the overall length.
In a very competitive field, CE has been shown to give as fast, reproducible and reliable DNA separation as polyacrylamide and agarose slabs. As traditional gel electrophoresis, the CE separation mechanism is that of molecular sieving (CGE).
To achieve length polymorphism separation, cross-linked gel-filled columns have been used (typically 26% T and 3-6% C polyacrylamide). However, gel-filled columns generally have a short lifetime because of localized overheating, irreversible binding of DNA strands to gel and capillary clogging. As an alternative, noncrosslinked gel sieving media have been introduced, based on water solutions of linear polymers, such as methylcellulose or linear polyacryla-mide, at a suitable concentration. In these media, the mutual entanglement of target and matrix molecules has a sieving effect and leads to separation by fragment length.
Fresh noncrosslinked refills are available by simply flushing new buffer into the capillary. On the other hand, noncrosslinked gel sieving systems usually show a weaker resolving power and lower efficiency, compared to crosslinked gel capillaries.
Noncrosslinked gels in CGE systems have been applied to problems of forensic genetics. The sieving buffer system was based on 0.5% (w/v) hydroxyethyl-cellulose in 100 mM Trisma base and 100 mM boric acid, adjusted to pH 8.7 with cesium hydroxide; the intercalating agent ethidium bromide and EDTA were added at suitable concentrations. Phenyl-methyl-coated silica capillaries (70 cm x 100 um i.d.) were used.
Few but important specific problems arise from the coupling of CE and PCR. In short, the presence of inorganic ions at high concentrations and excess primers in the PCR mixture has a detrimental effect on the electrokinetic injection of DNA molecules, as the small inorganic anions are injected preferentially, owing to their more favorable mass/charge ratio. Therefore, the PCR samples have to be ultrafiltered and/or dialyzed. Furthermore, optimum polymer concentration is a crucial point. A concentration 0.51.75% has a positive effect on resolution of small fragments, likely reflecting a reduction in effective pore size, although other interactions cannot be excluded. DNA fragments in the range 150-400 base pairs may require even higher concentrations of the polymer, which, on the other hand, has a negative effect on the resolution of higher molecular mass fragments. Besides, the upper limit of polymer concentration is determined by the viscosity of the arising solution, as highly concentrated polymer solutions may not be pumped into the tiny separation capillary. Increasing ethidium bromide concentration from 0.635 to 6.35 mM, reportedly, improves resolution, but higher concentrations have a deleterious effect on the column performance, particularly if left for longer periods in the column.
Noncrosslinked gels have been used for sieving CE with UV detection for the analysis of two genetic markers, namely D1S80 (repeat unit of 16 base pairs) and SE 33 (repeat unit of 4 base pairs) by using 0.635-1.27 uM ethidium bromide in the background buffer containing 0.5% hydroxyethylcellulose.
By using a similar noncrosslinked gel CGE system, LIF detection to PCR amplified DNA has been applied. Since DNA has only a weak native fluorescence, PCR products must either be tagged using fluorescent primers, or stained with fluorescent dyes. The use of fluorescent dyes intercalating DNA is reportedly more sensitive than PCR tagging because a greater number of fluorescent molecules can be introduced. Also, DNA intercalation is known to improve the separation, by increasing the rigidity of the fragments. YO-PRO-1 was adopted for DNA intercalation because of its strong binding constant to DNA and little intrinsic fluorescence, when not bound to DNA. The application of noncrosslinked gel CGE with LIF detection to the analysis of polymerase chain reaction-amplified DNA fragments showed an excellent sensitivity, about 500-fold better than with UV detection. With this procedure, the alleles from a number of loci of interest in forensic genetic typing were investigated with success.
After these pioneering articles, numerous papers have been published on noncrosslinked CGE-LIF analysis of forensic DNA, particularly after the recent introduction of fully automated instrumentation for DNA fragment analysis and DNA sequencing. The most advanced approaches make use of multiwave-length fluorescence detection and/or multicapillary systems to increase reliability and productivity, respectively.
Conclusion
Discussing the admissibility of new science or science tools in the USA courts it has been stated:
Given the economical and efficiency advantages of CE for forensic science and the judicial process, expert testimony based on CE analysis can be anticipated. The legal criteria of Daubert, as long as they are met by the scientific community, will allow CE and other legitimate scientific processes into evidence as acceptable expert testimony. (Kuffer eta/. 1996)
In fact, the reputation of CE as a sound technology in several applied analytical sciences, including forensic science, is now well established.
Versatility (reflected in a variety of application
fields) is probably the quintessential feature of CE, which is indeed day after day endorsing its image as not merely ‘a new analytical tool’, but a new ‘dimension’ in separation science. Of course, CE requires specific experience and new skills, which are still scarce in the forensic science environment, to the increasing cohort of new adepts.
Complementarity, the possibility of interfacing with other established techniques (spectroscopic, chromatographic, mass-spectrometric, electrophore-tic) and the ability to analyze tiny amounts of samples are other outstanding features of CE of particular relevance in the forensic field.
On the other hand, CE has a tremendous innovation potential (e.g. immunochemical multianalyte drug assays, CE on a chip) that requires a really openminded, interdisciplinary approach, which is still rare in the quite conservative field of forensic science. Undoubtedly, in many areas of potential application of forensic CE, e.g. in forensic toxicology, the electrophoretic background of scientists is, generally speaking, poor, whereas other areas, such as forensic biology, have little familiarity with instrumental analysis.
For this reason, seminal activity on CE and personnel training are priorities for the near future.
In conclusion, we believe that, when forensic scientists will be more familiar with the new concepts of CE, this fascinating technique will become a ‘normal’ tool in the hands of forensic scientists for everyday work and will no longer be regarded as an exotic technique closer to academia than to the ‘real world’.
