Introduction
To secure the best value from this article it is recommended that the article entitled ‘Mechanism of Explosion’ be read first.
The first use of explosives for commercial enterprises dates back to the seventeenth century, for example for mineral mining in Hungary, tin mining in Cornwall in the UK and civil engineering in France with the building of the Languedoc tunnel. In all of these cases the explosive used was black powder. Even today black powder still finds limited commercial use for example in slate quarrying where the shattering effect of a high explosive would be too damaging to the fragile slate.
The true potential of explosives for mining and quarrying began with the development of nitroglycerine-based explosives in the nineteenth century and the advent of the detonator. This created a much more efficient method of using chemical energy both in breaking rock and for lift and heave in trenching or cratering to move earth. These dynamites or gelignites were the mainstay of commercial explosives until the 1950s at which time explosives based on ammonium nitrate began to develop. This has led to the development of slurry and emulsion explosives and these are now displacing the nitroglycerine explosives in the commercial sector.
In comparison to military high explosives, commercial explosives are driven by cost. This is because the explosive is a significant component in the costs of a commercial enterprise such as quarrying or tunneling. It may be more cost effective, particularly in recent times, to use mechanical methods instead of explosives. The Channel Tunnel between the UK and France was excavated without the use of any explosives. Furthermore, in relation to costs, as the shattering ability or brisance of the explosive increases, so does the cost of the explosive. This means that there is a need for a range of explosives with different properties, particularly their detonation pressure but also their power, that is gas expansion effects.
Performance Parameters Indication of performance
For commercial explosives the ability to create lift and heave (power) is usually described by strength. This is a comparison of a particular explosive with a standard. The comparison may be with the same weight (mass) or the same volume of the standard giving the ‘weight strength’ or ‘bulk strength’, respectively. For nitroglycerine-based explosives, strength is usually compared to blasting gelatine, the most powerful of the type. More recently, strength may be compared to other standards and thus the literature must be scrutinized carefully to assess the actual performance. The method of conveying the information either in the literature or on the wrapping or container of the explosive charge is to quote the strength as a percentage, e.g. 80% strength.
The manufacturer rarely gives the detonation pressure; however, the literature will give the density and the detonation velocity. Together, these will indicate the shattering ability as high velocity of detonation and high density (relative) give high detonation pressure. This is indicated in the description of the various types of explosive in the following text.
Fume characteristics
One of the hazards of using explosives in confined spaces such as tunneling is the production of toxic fumes. Commercial explosives are mixtures of various ingredients and it is possible to vary the ratio of fuel elements to available oxygen. This means that the manufacturer can create explosive formulations that minimize the production of carbon monoxide thus making them much safer to use in confined situations.
For some explosives it is possible to create toxic products by having an excess of oxygen relative to the fuel that is present. This is particularly true for ammonium nitrate/fuel oil where a deficiency of fuel may lead to the production of oxides of nitrogen, seen as a brown gas cloud after detonation.
Water resistance
Another property of commercial explosives that is important to the user is the ability to use the explosive in the presence of water, for example in wet boreholes. Thus, the details provided for a particular product would state the water resistance from ‘none’ to ‘excellent’. This water resistance may be due to the packaging in more recent times with the advent of plastic containers. However, in earlier times it was a property of the actual formulation. For example, ammonium nitrate/fuel oil as a loose material has no water resistance, whereas the gelignites with a high nitroglycerine content have excellent water resistance.
Shock sensitivity
An important factor in the use of commercial explosives is the knowledge of the ability to reliably detonate the explosive charge with a detonator. This is known as detonator or cap sensitive. The formulations containing nitroglycerine are detonator sensitive; however, many of the bulk borehole charges are not. These include ammonium nitrate/fuel oil and certain slurries and emulsions. However, it is possible to formulate slurries and emulsions that are detonator sensitive.
For charges that are not detonator sensitive, a booster (or primer) is employed. This is an explosive charge that will reliably detonate from a detonator and amplify the shock wave to then detonate the insensitive column of explosive.
Critical diameter
Critical diameter is the minimum diameter of a bare charge of explosive that will sustain a detonation shock wave. For military explosives this value is very small, often 1-2 mm. However, for commercial explosives the value can be much greater, perhaps as much as 50 mm. This requires a matching of explosive type to borehole diameter. In practice, the manufacturer will supply cartridged explosives in diameters that are guaranteed to detonate fully. If, however, the explosive is to be bulk loaded into a borehole it is imperative that the hole diameter is well above the critical diameter for the explosive.
Nitroglycerine-containing Explosives
From the mid-nineteenth century to the mid-twentieth century, nitroglycerine (NG) (Fig. 1) was the most important energetic ingredient and sensitizer for commercial explosives. The early developments were attributed to the Nobel family in Sweden with Immanuel Nobel being the first to realize the potential of nitroglycerine and then his son Alfred who has now been immortalized through his efforts in developing modern explosives and the introduction of the Nobel Prizes. The oily liquid is manufactured by the reaction of glycerine (glycerol, propane-1,2,3-triol) with a mixture of concentrated nitric and sulfuric acids, during which the temperature must be controlled carefully to avoid a rise in temperature that could cause a runaway decomposition of the explosive that has formed resulting in an explosion. Modern synthesis uses a continuous nitration process where the maximum quantity of explosive in the reaction vessel is limited.
Pure nitroglycerine has a melting point of 13°C and it is undesirable for the explosive to freeze when mixed in a formulation. Partly thawed nitroglycerine is dangerously sensitive; this is thought to be due to the presence of triclinic crystals that may rub together if the charge is handled. To overcome this, another explosive molecule, ethyleneglycoldinitrate (EGDN, nitroglycol) (Fig. 1) is mixed with the nitroglycerine to lower the freezing point. EGDN is manufactured at the same time as the nitroglycerine by using a mixture of glycerine and glycol (ethane-1,2-diol) in the nitration reaction. The ratio of these explosive types is usually around 50/50 and lowers the freezing point of the mixed liquid explosives to around — 10°C. Pure nitroglycol freezes at — 22°C.
The range of explosives described below is manufactured worldwide; however, in recent years the development of commercial explosives that do not contain nitroglycerine is causing a serious decline in the use and therefore production of this type of explosive. It is estimated that in the very early part of the twenty-first century, the production of dynamites will cease.
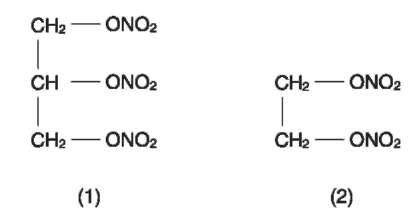
Figure 1 The structure of nitroglycerine (1) and ethyleneglycoldinitrate (2).
Dynamite explosives
This group of explosives was developed from the original dynamite of Nobel that was simply nitroglycerine absorbed into kieselguhr, a dry powdered clay. This significantly reduced the extreme sensitivity of the nitroglycerine to shock initiation. There were two major developments that followed: (1) the addition of nitrocellulose (NC) that gelled with the nitroglycerine (and nitroglycol as mentioned above); (2) the inclusion of a fuel/oxidizer mixture in the formulation. The use of nitrocellulose allowed the liquid nitrogly-cerine/nitroglycol to form a gelatinous mixture giving useful physical properties and minimizing the separation of the liquid explosives from the mixture. Also, it significantly reduced the sensitivity of the pure liquid explosives. This led to a range of explosives known as gelatine dynamites or gelignites with the NC content being approximately in the ratio 1:20 with the NG/EGDN. The original dynamite as described above is no longer found.
The use of a fuel/oxidizer mixture gave the extra dynamites or extra gelatine dynamites although it should be noted that these are often simply called gelignites. The fuels are usually cellulose based, such as sawdust or wood meal (fine sawdust), and the oxidizer is either sodium or, more usually, ammonium nitrate. In some countries the term ‘straight gelatine dynamite’ may be used to differentiate the use of sodium rather than ammonium nitrate and ‘ammon’ or ‘special’ to denote the use of ammonium nitrate.
This allows a large range of explosive formulations to be produced depending on the ratio of gelled nitroglycerine/nitroglycol to fuel/oxidizer mixture. Those with a reasonable gelled nitroglycerine/nitro-glycol content are rigid in form and are the true gelatine explosives. They can be deformed by pressing with a tamping rod when loading shot holes. As this ingredient is reduced in the formulation then the consistency becomes more crumbly. These are known as semigelatines and will have reduced performance. Finally, the content may be reduced so much that the consistency is a powder; at this stage it is unlikely that nitrocellulose is present. These low performance explosives are the nitroglycerine powders. In some countries it is possible to find formulations that have the same NG/EGDN content as a gelatine or semi-gelatine explosive but without the NC as a gelling agent. Confusion may occur as these have been called ‘straight’ dynamites in some texts.
Other additives may be found in these formulations. Both barium sulphate and manganese dioxide can be added to increase the density and achieve maximum velocity of detonation. Sodium chloride and calcium oxalate are added to act as flame suppressants (see below) and often naturally occurring gums are present to aid consolidation of the mixtures. Calcium carbonate may be added to act as a stabilizer to minimize the risk of autocatalytic decomposition of the nitroglycerine/nitroglycol. On rare occasions, dinitrotoluene may be present.
Permitted explosives
When blasting in coalmines there is always the risk of secondary explosions and fire from ignition of either methane/air or suspended coal dust/air mixtures. To overcome this problem permitted explosives were developed. These must pass rigorous national testing to insure that such ignitions cannot occur and are categorized for particular uses. They are given designations such as P1-P4/5 in the UK.
The source of ignition for the fuel/air mixtures that may be present could be one of the following:
1. A long lasting flame as would be produced from a black powder explosion;
2. A secondary burn of hydrogen/carbon monoxide produced by an explosive that had a negative oxygen balance;
3. An intense shock wave from a high detonation pressure explosive causing shock heating of the surrounding air.
To overcome these possible mechanisms formulations have been devised that have relatively low shock pressure and a positive oxygen balance. Furthermore, sodium chloride is added in significant quantity, as much as 30% by weight, as this acts as a flame suppressant by interfering with the flame propagation process.
Performance
Nitroglycerine is a very powerful explosive producing an energy release of 6275 J g_1 and 740cm3g_1 of gas. This ranks with the highest performing explosive molecules for power output. Nitroglycol has an even higher energy output at 6730 J g_1 but the same gas production. It is clear that a high percentage of these ingredients will produce an explosive with good lift and heave. Also, the velocities of detonation and densities suggest a high detonation pressure. However, as discussed above it is usual to find these explosive liquids absorbed into a nitrate/wood meal mixture and this will significantly reduce the density and velocity of detonation.
The most energetic formulation of this group of explosives is blasting gelatine, which contains 9294% nitroglycerine/nitroglycol together with 6-8% nitrocellulose. This is used as the standard against which the strength of other gelatine dynamites is measured. This explosive is rarely used in practise, as the level of performance is hardly ever required. As the percentage of nitroglycerine/nitroglycol decreases the detonation pressure falls quite rapidly. However, although the power also falls the percentage reduction is not as great as for the detonation pressure.
Typical percentages of nitroglycerine/nitroglycol for the various types described above will be:
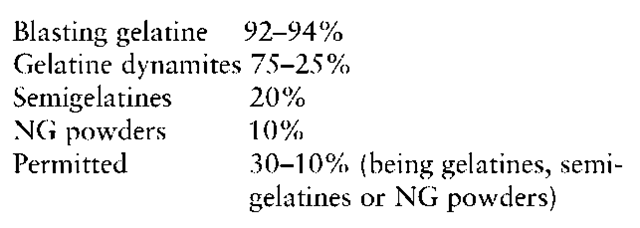
The weight strength, bulk strength, density and velocity of detonation for typical examples of the various types are given in Table 1. The velocity of detonation is the maximum as achieved by a strong shock initiation. These types of explosives have an unusual feature if initiated with a relatively weak shock. They will detonate with a velocity of detonation of ~2500ms — 1 irrespective of the NG/EGDN content. Thus a gelatine, semigelatine or NG powder can have two quite different values for a stable velocity of detonation.
Table 1 also shows that the bulk strength often is lower than the weight strength. This is because in those cases the density of the explosive is much lower than the blasting gelatine standard.
Military Dynamites
It should be noted that in the United States, military dynamites are not based on nitroglycerine but contain trinitrotoluene (TNT) and cyclotrimethylene trinitra-mine (RDX) as the explosive molecular components.
Ammonium Nitrate-based Explosives
Ammonium nitrate fuel oil (ANFO)
Any carbonaceous material mixed with ammonium nitrate (AN) will produce a possible explosive mixture. In fact AN as a pure material is not classified as an explosive for transportation and storage unless it contains >0.4% carbonaceous material. However, there have been many apparent incidences of pure AN being transported in bulk that has caught fire and ultimately transitioned to a detonation. In all cases where an explosion followed an AN fire, the mass of AN was confined, for example in the hold of a ship, and it is contested was almost certainly contaminated with combustible materials to some extent. The infamous incident at Oppau, in the Rhineland of Germany, was caused by the explosive blasting of an approximately 50/50 mixture of AN and ammonium sulfate. Although it was found that there had been around 20 000 blasts carried out previously, a procedure used to break up the hard consolidated mass of AN/ammonium sulfate, in this instance it is likely that the condition of the material had altered from the norm and probably contained less moisture and was of slightly lower density. The ensuing explosion killed 500, injured 1900 and caused damage in a town 1.5 km distance. Most of Oppau was completely destroyed. The explosion was estimated to be equivalent to around 500 tonnes of TNT.
Ammonium nitrate (NH4NO3) has been manufactured as a fertilizer at least since the turn of the twentieth century. Developments in the 1950s led to a form of AN known as prills. These were small spherical or ovoid beads, 2-3 mm in diameter, manufactured by passing concentrated AN solution droplets down a drying tower against a rising stream of hot air. Control of the conditions gave prills of differing densities. The crystal density of AN is 1.7gcm—3. For agricultural use a high density prill is favorable at ~1.1gcm—3; however, for use in the manufacture of an explosive a lower density is better at 0.8-0.9 gcm—3. This is because the porosity of the lower density material allows the liquid fuel (fuel oil or diesel) to absorb readily into the prills. Thus, an intimate fuel/oxidizer mixture is achieved. These lower density prills are somewhat prone to physical breakage, which at first appeared to be a disadvantage. However, with some modern methods of use it may actually be advantageous as described below.
Table 1 Performance figures for nitroglycerine-based explosives
| Explosive type | Weight strength vs BG (%) | Bulk strength vs BG (%) | Density (g cm—3) | Velocity of detonation (ms—1) |
| Blasting gelatine | 100 | 100 | 1.6 | 7500 |
| Gelatine dynamite | 80-65 | 80-65 | 1.5-1.4 | 6600-5500 |
| Semigelatine | 65-50 | 60-50 | 1.3 | 6000-5000 |
| NG powder | 80 | 65 | 1.15 | 4000 |
The commercial explosive is known as ANFO (AN fuel oil) and the correct proportions of AN to diesel, a commonly used fuel oil, are 94/6 based on pure AN. If it is known that the AN is not pure then the diesel percentage should be adjusted to give the same ratio of the AN content to the diesel. It is important to ensure that the fuel/oxidizer balance is correct as variance may give large quantities of carbon monoxide if fuel rich, or of nitrogen dioxide if oxidizer rich. Most explosives manufacturers will sell both ready-made ANFO and bags of AN prills for the user to manufacture the ANFO on site. For large-scale use it is mixed on site in a purpose-built vehicle and poured directly into vertical boreholes. ANFO can be loaded into horizontal or vertically inclined boreholes by pneumatic loading. This is carried out at a pressure of ~ 4 bar which causes the density to rise from the 0.8-0.9gcm-3 to ~1.1gcm-3. This provides an explosive fill that has more power and a higher velocity of detonation.
Performance of ANFO
ANFO is a very insensitive explosive, which makes it extremely safe to use. However, it is not detonator sensitive and therefore requires the use of a booster to provide reliable detonation. When poured loose into a borehole or pneumatically loaded, there will be maximum filling of the hole. This does not occur when cartridged charges are used.
On the other hand, ANFO has no water resistance unless packaged in waterproof containers. It cannot be loaded directly in to wet boreholes as this makes the column of explosive insensitive even to a booster. Also, ANFO has a large critical diameter and is not likely to propagate a detonation shock wave successfully in boreholes of less than 50 mm in diameter. Figure 2 indicates how the velocity of detonation varies with borehole diameter. As the value increases so will the shock pressure. A commonly used borehole size is 100 mm (4 inches).
Typical values quoted for the weight strength and bulk strength of ANFO are 70% BG (blasting gelatine) and 30% BG, respectively. The bulk strength is so low because of the very low density of poured ANFO. Addition of aluminum powder increases the energy in the mixture and gives weight and bulk strengths of 77% BG and 40% BG, respectively. The shock pressure of these explosives will be in the region of 10-30 kbar. The higher values will be for large diameter boreholes as the velocity of detonation is higher or for the pneumatically loaded ANFO where the density is higher. A typical value for velocity of detonation for poured ANFO in a standard borehole of 100mm would be <3000m s_1.
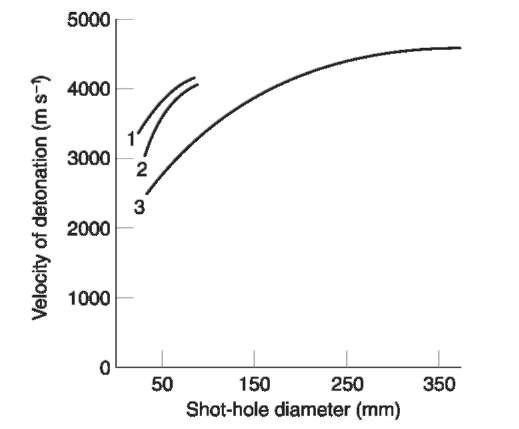
Figure 2 Velocity of detonation for ANFO (poured and pneumatically loaded) versus borehole diameter. 1, Pneumatically loaded, 1.1 gcm~3; 2, pneumatically loaded, 0.95gcm~3; 3, poured ANFO, 0.8gcm~3.
Slurry explosives
A slurry explosive is based on AN as oxidizer mixed with a fuel as with ANFO. However, they have as part of the formulation a significant percentage of water, usually 10-25% by weight. The water, together with the presence of guar gum or equivalent as a gelling agent, produces an explosive superior in many ways to ANFO. The basic formulation is ammonium nitrate solid together with either a carbonaceous fuel or aluminum powder or both held in suspension in a gelled saturated solution of ammonium nitrate. Over the years many types of carbonaceous fuels have been used, ranging from solid hydrocarbons, powdered coal, carbohydrates (e.g. sugars), bagasse (sugar cane cellulose) to paint-grade aluminum. When aluminum is present in the mixture it is usual to include an additive to buffer the pH against the acidity caused by the presence of the ammonium nitrate. The pH is held at about 5 to prevent reaction with the aluminum that would evolve hydrogen.
To provide sensitivity to shock initiation it is usual to introduce very small (40-50 um) air pockets or bubbles, which act as centers that create hot spot initiation when a shock wave passes. This is done in one of several ways the simplest of these being to use a beating process and the air bubbles are trapped as the gel sets. Other methods are to add glass or plastic microballoons or expanded polystyrene beads. These are favored if it is thought that the pressure in a borehole may squeeze the explosive increasing the density and reducing the number of air pockets or if the shock pressure from an adjacent borehole creates a similar effect. A recent chemical reaction method has been used to create the bubbles. This is the reaction between sodium nitrite and acetic acid which produces nitrogen gas bubbles. The presence of aluminum powder also leads to hot spot creation and in some cases actual explosive molecular types are added such as smokeless powder (propellant) or 20-mesh TNT. The most recent developments have led to the use of nitrated chemical sensitizers that introduce both sensitivity and fuel, examples being isopropyl nitrate or the now favored methylamine nitrate.
Until relatively recently, the manufacture of these complex mixtures could be achieved only under factory conditions. Thus, slurry explosives were supplied cartridged in flexible plastic tubing similar to that used to package meat products such as liver sausage. Current cartridge sizes have diameters of 25-200 mm and weigh between 200 g and 5 kg. In more recent times systems have become available for on-site manufacture and mixing trucks are available that manufacture and pump the product directly into the borehole. A further development has been the use of blends of slurry with ANFO (see below).
Performance of slurry explosives
At first, slurry explosives were detonator insensitive and required a booster as for ANFO. Developments have now led to compositions that are detonator sensitive, but the majority still require boosting. Due to their physical nature as water-containing gelled systems they are quite insensitive to accidental initiation. The use of gelling agent gives these explosives a reasonable degree of water resistance if loaded unpackaged into a wet borehole and thus is a superior explosive to ANFO. Most slurry compositions have reasonably large critical diameters and are employed in boreholes greater than 75 mm in diameter although as noted above, the smallest size is a 25 mm diameter cartridge.
As explosives they are superior to ANFO due to a large extent to the higher densities that are achieved. Densities are in the range of 0.9-1.4 gcm—3 and this, together with the higher energies gives velocities of detonation in the range 3500-5500 m s —1 and this will give shock pressures in the range 50-100 kbar. Depending on the formulation, weight strengths are in the range of 50-100% BG and bulk strengths from 30-75% BG. The much higher density than ANFO produces bulk strengths that are relatively not as low compared to the weight strength as is seen for ANFO.
Emulsion explosives
At first sight the formulation of an emulsion explosive appears very similar to a slurry being based on ammonium nitrate, water, hydrocarbon oil, aluminum powder and a sensitizer. However, there is a fundamental difference in the physical nature of the two explosives. A slurry has a continuous aqueous phase, whereas the emulsion consists of aqueous droplets containing the ammonium nitrate held in a true emulsion with a hydrocarbon continuous phase. The hydrocarbon layer may be as little as 3 um in thickness. Depending on the properties of the hydrocarbon, from a mobile oil to a wax at ambient temperatures, the consistency of an emulsion explosive ranges from toothpaste to putty.
Other than this, there are a number of similarities between emulsions and slurries. The sensitization is achieved by exactly the same methods as described above. Both factory cartridged and truck-mixed versions are used and the critical diameter considerations are about the same although those with the higher densities are detonator sensitive and maintain the detonation in small diameter, e.g. 25 mm.
A current technique in the use of emulsion explosives is to use a mixture with ANFO. This fills the voids in the standard ANFO fill creating not only a denser material but also one of higher energy. The mixtures range from 70/30 emulsion/ANFO to 30/70 emulsion/ANFO. The former can be pumped but the latter is like thick porridge and is augered into the boreholes.
Performance of emulsion explosives and emulsion/ANFO blends
The range of emulsion explosives is very similar to the slurries in explosive performance for velocity of detonation, weight strength and bulk strength. However, advantage is gained by using the blend technique. Table 2 indicates the change in density and energy between ANFO, an emulsion and a typical blend. The velocity of detonation tends to be higher for the blend than either component and can approach 6000 m s— 1 at a density of ~1.4gcm—3.
Miscellaneous types
There are two types of ammonium nitrate-based explosives that are available but are peripheral to the mainstream commercial production of explosives. In one the AN is mixed with the liquid nitro-methane (CH3NO2), perhaps up to 15% of the liquid to produce an explosive with a velocity of detonation of ~ 5500 m s— 1 and shock pressure of ~ 90 kbar. In the other the AN in mixed with hydrazine (N2H4)to produce a trade product known as ASTRO-PAK or
Table 2 Performance comparison between ANFO, an emulsion and an ANFO/emulsion blend
| Property | ANFO | Emulsion | ANFO/emulsion blend |
| Density (g cm”3) | 0.8 | 1.25 | 1.3 |
| Energy (kj g”1) | 3.8 | 2.9 | 3.6 |
| Energy (kj cm”3) | 3.13.6 | 4.5 | |
| Velocity of detonation (m s”1) | 3000 | 4500 | 5500 |
Astrolite T. The explosive is a liquid with a density of ~ 1.4 gcm”3 and a velocity of detonation of 8000 m s”1 if initiated by a strong shock. This will give a detonation shock pressure of > 200 kbar and thus the ability to break metal.
Detonating Cords
Essentially, detonating cord is a plastic tube, sometimes reinforced with wound or woven fibers and filled with powdered explosive. It is used to transmit a detonation shock wave for multiple charge initiation, that is to link charges together, or to lead a detonation shock wave from a detonator to a booster that may be in a borehole. Also, it may be used as an explosive charge in its own right.
There are three parameters relevant to the description of a particular cord. One is the tensile strength, which is important if any load may be applied to the cord. This tends to be relevant only for use in oil wells. The second is the type of explosive used to fill the cord and the third the loading of explosive per unit length. Obviously, as the loading increases so will the diameter of the cord. The smallest commercial cord contains 1 g m”1 of explosive and the largest 100 g m”1.
In general use, cords containing 5-8 g m” 1 are used to lead a detonation to a booster or to initiate shock tube (see below). The cords used for linking multiple charges contain 10-12 g m”1 and the cords with 40 g m” 1 upward are used for engineering operations such as presplitting of rock or for demolitions.
The general explosive filling is pentaerythrytol tetranitrate (PETN) as this explosive has a small critical diameter of ~ 1 mm and thus will propagate a detonation shock wave reliably in very small columns. However, when cords are used in oil wells there may be a problem associated with the temperature in the deeper wells. In down-hole operations it is necessary to have the explosive charges in the hole for some time before firing. The cords filled with PETN can be used at a maximum temperature of 135°C for 1 h or 121°C for 24 h. For higher temperatures different explosives are used, structures of which are given in Fig. 3 and approximate maximum operating temperatures in Table 3.
Boosters (Primers)
As discussed above, many commercial explosives are not detonator sensitive. Reliable initiation is achieved through the use of booster charges that provide an intense shock wave to the column of explosive in the borehole. The most commonly used explosive in a booster is Pentolite, a 50/50 mixture of PETN and TNT. This has a detonation shock pressure of ~ 250 kbar and reliably detonates ANFO, slurries and emulsions.
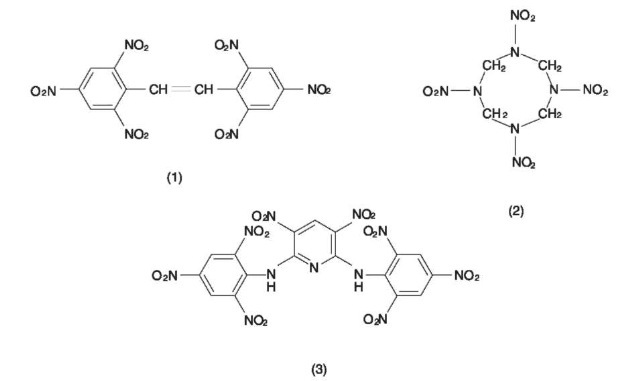
Figure 3 Structures of some explosives used at high temperatures. 1, hexanitrostilbene; 2,
Table 3 Approximate maximum operating temperatures for prolonged residence times
| Explosive | Maximum operating |
| temperature (°C) | |
| Pentaerythrytoltetranitrate (PETN) | 125 |
| Cyclotrimethylenetrinitramine (RDX) | 165 |
| Cyclotetramethylenetetranitramine (HMX) | 180 |
| Hezanitrostilbene (HNS) | 300 |
| 3,5-dinitro-2,4-di(picrylamino)pyridine (PYX) | >300 |
Detonators
Standard demolition detonators
For commercial use it is normal for electric detonators to have a time delay from the firing current initiating the fuze head to the detonator providing the shock output. This is done by introducing a delay element consisting of a column of pyrotechnic between the fuze head and the lead azide pellet. The concept of delay detonators is to allow one firing signal to be sent to multiple detonators that will initiate all the fuze heads; however, for different delay times this will give different firing times for the detonators. This reduces ground shock when quarrying by separating borehole detonations by intervals of ~25ms. In tunneling it allows some charges to fire first to create a face towards which the next row of charges firing some time later can work. Finally, for demolitions it is necessary for some parts of a structure to be blasted first giving a controlled collapse or a direction for toppling. There are two delay series; the millisecond series separated by 25 ms intervals up to 850 ms for some manufacturers and the half-second series usually up to a maximum of 6 s.
Delay detonators can be identified and are classified by the delay number stamped on the end of the detonator body or on a plastic or paper tag attached to the leg wires. This is usually a number that must be related to the literature of the manufacturer to ascertain the delay time. This literature will describe the color of the leg wires and the type of wire (iron, copper, etc) and the material for the body, normally copper or aluminum.
There are nonelectric detonators known as ‘plain’ detonators. These are essentially an electric detonator without the fuze head and associated leg wires. To initiate this a delay fuze is inserted into the open end of the tube and crimped in place with a special tool immediately before use. The delay fuze usually contains black powder and burns at a steady rate of ~ 300 mm in 40 s. The flash output from the fuze initiates the lead azide pellet and fires the detonator. These are commonly used for single shots such as breaking a large rock on the quarry floor or in tree stump removal.
Special initiating systems
There have been several examples of developments aimed at improving the safe use of detonators. The Magnadet detonator was developed in the UK by ICI Explosives Ltd as a system that was immune from induced current initiation caused by electromagnetic radiation. This problem exists for fuse head-containing detonators, as the majority will fire from a current as low as 0.5 amp. This can be induced if an electric detonator is used near a powerful radio, radar or under electric power cables. The Magnadet is a normal electric detonator with very short leg wires which form a continuous circuit with several loops wrapped around a ferrite toroid. The firing cable is not attached electrically to this circuit but passes through the hole in the toroid. When a special firing signal is sent down the firing cable it induces a current in the detonator circuit and fires the detonator.
Perhaps the most important development in recent years has been the shock tube detonator. This system relies on the initiating signal being transmitted to a detonator via a small hollow plastic tube ~2mm in diameter the inside of which is coated with a mixture of the explosive cyclotetramethylenetetranitramine (HMX) and aluminum powder at a loading of ~20mg m—1. This layer propagates a shock wave at ~ 2000 m s —1 which on reaching the detonator initiates the delay element if present or the lead azide pellet directly. There is so little explosive present that the tube is not disrupted and indeed could be held as the shock wave passes down the tube. The shock tube is factory fitted to the detonator and is available in various lengths. Systems are available to link tubes to create bunch firing. The advantage of this system is that it is completely immune to initiation from stray electric currents or induced current from electromagnetic radiation.
A recent development has been the introduction to the market of detonators that do not contain lead azide. It has been replaced with a finely powdered secondary explosive, PETN, which in a confined metal collar burns to detonation. The advantage of using a secondary explosive instead of the primary explosive, lead azide, is that the detonator is much more difficult to initiate accidentally. Trials in which rocks and weights were dropped on to both types of detonator demonstrated this insensitivity.
