Introduction
Ischemic heart disease (IHD) is one of the main groups within the class of cardiovascular diseases (CVD), and IHD is the most common single cause of morbidity and mortality in the Western world (Gaziano et al., 2006). According to the American Heart Association Statistics Committee one in three individuals has one or more forms of CVD (Rosamond et al., 2007).
The importance of inflammation in the pathogenesis of IHD, such as, acute myocardial infarct (AMI) and coronary artery disease (CAD), has long been established. A large body of evidence suggests that inflammation plays a key role in CVD, however, the mechanisms in the various stages of the pathological process is not completely understood (Carden and Granger, 2000). Inflammation is a complex of defensive mechanisms reacting to the entry of harmful agents into the organism or cells, in order to eliminate or repair damaged cells or tissue and to restore homeostasis. This broad definition indicates that inflammation does not only accompany infectious diseases, but also other conditions causing cell, tissue or organ damage.
The identified risk factors for IHD include both lifestyle and biological factors, such as smoking, high blood pressure, high cholesterol levels, obesity, and diabetes that all appear to exaggerate many of the vascular alterations elicited by ischemia and reperfusion. Diabetes and CVD often appear as two sides of a coin: on one side, diabetes has been rated as an equivalent of CVD, and conversely, many patients with established CVD suffer from overt or incipient diabetes (Ryden et al., 2007). The mortality from AMI is almost increased fivefold in diabetic patients compared with non-diabetics (Hansen et al., 2007) and diabetes and low-grade inflammation is closely related (Flyvbjerg, 2010). Obesity is seen at epidemic proportions all over the world, and is a significant risk factor for, and contributing factor to increased morbidity and mortality, most importantly from CVD and diabetes (Lavie et al., 2009a). Likewise, obesity is also associated with low-grade inflammation and CVD (Yudkin et al., 1999; Bastard et al., 2006).
Although the combination of traditional risk factors such as age, gender, lifestyle, dyslipidemia, hypertension and diabetes are well established for the prediction of cardiovascular mortality (e.g. the Framingham coronary risk score), these algorithms do not adequately differentiate individuals at moderate risk. Indeed, not all patients with CVD will have conventional risk factors and not all patients with risk factors will develop CVD (Khot et al., 2003). Both biomarkers of early disease and plaque instability have therefore been sought, and the development of new markers to diagnose and prevent CVD is an important public health goal worldwide. However, a recent report showed that the addition of a multi-marker score including 10 new markers to conventional risk factors added only a moderate increase in the ability to grade the risk in the general population (Wang et al., 2006).
Several inflammatory biomarkers have been shown to represent important cardiovascular risk factors, and this review will primarily focus on the complement system, the acute-phase reactant C-reactive protein (CRP), and the antimicrobial peptides: a-defensins. Whether these inflammatory proteins mediate IHD themselves or solely serve as markers of systemic inflammation and cardiovascular risk stratification is still intensely studied.
Inflammation and IHD
Growing evidence indicates that IHD is a broad syndrome with multiple pathogenetic and aetiological components, which may not be the same in all patients. Extensive literature supports the role of inflammation in IHD (Mehta and Li, 1999; Ross, 1999). Inflammatory cells, inflammatory proteins and inflammatory responses from vascular cells are all reported to play crucial roles in various stages of a number of CVD (Car den and Granger, 2000). Some of the inflammatory mechanisms of IHD include, among others, endothelial dysfunction, oxidative stress and vascular calcification, that all seems to play an important role for the development of cardiovascular disease.
Although timely restoration of blood-flow after a myocardial ischemic event is essential to prevent irreversible cellular injury, it is widely recognized that the outcome of tissue injury not only depends on the duration of the ischemic event, but also on reperfusion as a critical factor (Khalil et al., 2006). Paradoxically, the reperfusion exacerbates severe tissue damage, especially after longer periods of ischemia. The intensity of the inflammatory reaction in post-ischemic tissue can be so strong that the injury response to reperfusion can be manifested in distant organs (Carden and Granger, 2000).
The ischemia-reperfusion injury (IRI) results in a local and systemic inflammatory response characterized by the production of reactive oxygen species (ROS), leucocyte-endothelial cell adhesion, complement activation, endothelial leucocyte migration, increased micro-vascular permeability and decreased endothelial-dependent relaxation (Carden and Granger, 2000). Within minutes of reperfusion ROS are generated (Cannon, 2005), stimulating the release of cytokines and expression of adhesion molecules on damaged cells in reperfused tissue. Several hours after onset of reperfusion, neutrophils and other inflammatory cells are activated (Frangogiannis et al., 2002; Frangogiannis, 2007) and adhere to the damaged cell membranes (Zimmerman et al., 1990; Vinten-Johansen, 2004) for further enhancement of the inflammatory response. Thus, IRI poses major problems in the clinic, and effective therapies are required.
Inflammatory biomarkers
Recent research has been focused on identifying biomarkers, which alone or in combination with other risk markers could be useful in monitoring the treatment and as prognostic markers for future coronary syndromes and cardiac death in patients with IHD.
In 2001, a National Institute of Health working group defined a biomarker as "a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention (BiomarkersDefinitionsWorkingGroup, 2001). Biomarkers are traditionally specific proteins circulating in the body fluids that become altered as a consequence of disease progression or the effect of a therapeutic intervention and can be divided into different categories:
• Disease-predictive
• Diagnostic
• Prognostic
• Disease-associated
• Therapeutic efficacy.
A number of inflammatory biomarkers have been associated with cardiovascular diseases. Biomarker measurements can help explain empirical results of clinical trials by relating the effects of interventions on molecular and cellular pathways to clinical responses, thus providing an opportunity for researchers to gain a mechanistic understanding.
The complement system
The complement system is an innate, cytotoxic host defence that normally functions to eliminate pathogens and facilitates the clearance of damaged tissue and apoptotic cells. However, excessive activation of the system may lead to uncontrolled tissue damage. The relevance of complement activation in myocardial ischemia was already proposed more than four decades ago (Hill and Ward, 1971). The inflammatory mechanisms by which tissue injury after an AMI occurs has not been fully elucidated, but strong evidence obtained from animal models, as well as clinical studies, support the hypothesis of a role for the complement system in IHD (Bjerre et al., 2008).
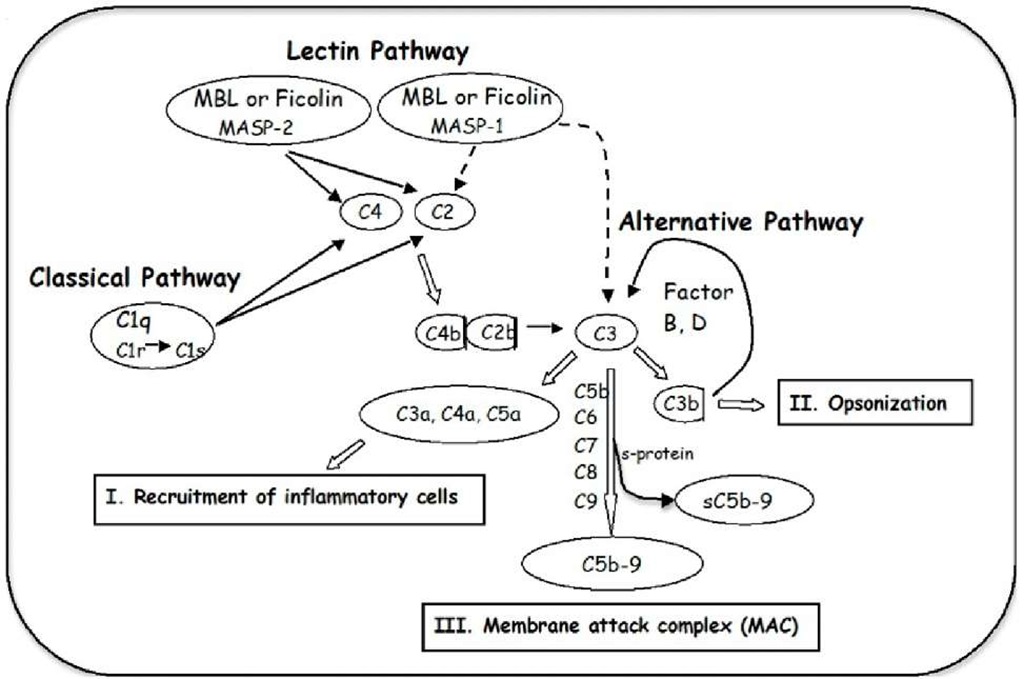
The complement system is a biochemical cascade, which helps clear pathogens from an organism, and is thus one part of a larger immune system. Three pathways of complement activation have been identified (Figure 1), known as the classical, the alternative and the lectin pathway. The classical pathway is initiated by C1q binding to antibody complexes (Cooper, 1985) whereas the alternative pathway is initiated by spontaneous and direct activation of C3 (Muller-Eberhard, 1988). The lectin pathway is initiated either through ficolin (M-, L- or H-ficolin) (Matsushita et al., 2001; Frederiksen et al., 2005), by pattern recognition of N-acetyl-glucosamine-rich polysaccharides or through mannan-binding lectin (MBL) binding to certain carbohydrate structures (Ikeda et al., 1987; Thiel et al., 1997; Holmskov et al., 2003).
Activation of the complement system promotes three main biological activities (Walport, 2001): I) recruitment of inflammatory cells by anaphylatoxins (C3a, C4a, and C5a), leading to accumulation of activated polymorph nuclear leukocytes directly involved in tissue destruction, II) opsonisation of pathogens for phagocytosis by the generation of C3b, and III) lysis of the pathogen by the generation of a membrane attack complex (MAC, C5b-9) penetrating the cell membrane. The loss of membrane integrity destroys the ability to control the concentration of salt within the cell and the cell is killed due to this osmotic instability. MAC formed in the absence of target membranes binds to S-protein, which inhibits the membrane-damaging effect, and creates a stable non-lytic soluble C5b-9 form (sMAC, sC5b-9) (Fosbrink et al., 2005).
Fig. 1. The complement system can be activated through three pathways, which merge at the cleavage of C3, leading to the effector mechanisms: I. recruitment of inflammatory cells, II. opsonisation and III. the generation of the membrane attack complex (MAC, C5b-9) for cell lysis.
Role of complement in endothelial function
Ischemia and reperfusion are potent activators of the complement system and both clinical and experimental studies in different organs have shown local deposition of complement (Riedemann and Ward, 2003; Arumugam et al., 2004). It has traditionally been assumed that the liver is the source of complement proteins that participate in these events, but complement proteins are produced in several organs of the body, including the heart and the endothelium.
The human heart expresses mRNAs, translated into proteins, for all of the components of the classical complement pathway (Yasojima et al., 1998b). This production is up-regulated in areas of myocardial infarct, and the classical complement pathway was found to be fully activated on injured myocardial tissue. In addition, production of C3 and C9 mRNAs and their protein products was significantly increased in isolated rabbit heart after reperfusion injury, and the production by heart in this circumstance substantially exceeds that of the normal liver (Yasojima et al., 1998a).
Endothelial cells also represent one of the extrahepatic sources of complement components and regulators. Due to the strategic position along the surface of the vessel wall, they may supply both the circulating blood and the extravascular fluids with these proteins. Most of the information on the production of complement components by endothelial cells has been obtained using human umbilical vein endothelial cells (HUVECs). HUVECs cultured in serum free media were reported to synthesize functional C3, C5, C6, C8, and C9 and assembling of the MAC complex was found, indicating that C7 was produced as well (Johnson and Hetland, 1991). The C7 production was later confirmed (Langeggen et al., 2000) and a small production of C5, C6, C8, and C9 demonstrated by RT-PCR and Northern blot was reported (Langeggen et al., 2001). Activation of the complement cascade leads to the formation of the C5b-9 complex on the cell surface that can cause cell death. However, when the numbers of C5b-9 molecules are limited on host cell membranes, it can activate signalling pathways leading to cell cycle activation and cell survival (Niculescu and Rus,
2001).
The reduction in the capacity of the endothelium to maintain the homeostasis leads to the development of pathological inflammatory processes and vascular diseases. An intact endothelium is a fully biocompatible surface that is not recognized by the complement system. However, blood contact with a damaged endothelium will lead to a certain degree of activation of the defence system. Complement activation results in the formation of several biological active components including C3a, C5a, iC3b, and C5b-9. In addition, to stimulate inflammatory response C5a have been reported to induce production of chemokines and cytokines (Czermak et al., 1999).
Mechanism of complement in IHD
Cardiovascular risk factors (e.g., hypercholesterolemia, hypertension, smoking, diabetes, stress) cause oxidative stress that alters the endothelial cell capacity (Esper et al., 2006; Kyrou and Tsigos, 2007), and thus dysfunction of the endothelium has been implicated in the pathophysiology of different forms of CVD (reviewed by (Endemann and Schiffrin, 2004)). Human endothelial cells have been shown to generate reactive oxygen intermediates in response to hyperglycaemia and lead to dysfunction in type 2 diabetic patients (Bellin et al., 2006). Endothelial dysfunction has been shown to have a prognostic value in patients with chest pain (Neunteufl et al., 2000) and both C-reactive protein (CRP) and C3a were found to be elevated in patients with unstable angina pectoris (Kostner et al., 2006). Elevated plasma levels of C3 was independently associated with MI after accounting for traditional cardiovascular risk factors and CRP in patients with MI, whereas the association of CRP was dependent of C3 (Carter et al., 2009).
A number of in vitro studies using HUVECs have shown that under anoxic conditions the endothelial cells become activators of the complement system. Collard and co-workers showed increased MBL, C3, and iC3b deposition after hypoxia/reoxygenation (Collard et al., 2000; Collard et al., 2001). MBL deposition was attenuated in the presence of MBL ligands indicating the presence of MBL neo-epitopes on the surface of the endothelial cells. Apoptotic endothelial cells deposited C1 and C3d, thus activating the complement system (Mold and Morris, 2001). Experimental studies in porcine and rabbits reported that complement activation attenuates endothelium-dependent relaxation (Stahl et al., 1995; Lennon et al., 1996). The role of complement was mediated by the formation of C5b-9, but not through the generation of the anaphylatoxin C3a and C5a.
Hill et al. were the first to report a role for the complement system after an ischemic event in a rat model of AMI (Hill and Ward, 1971). The association between AMI and complement activation was later confirmed in baboons showing deposition of complement factors in the infarcted tissue (Pinckard et al., 1980; McManus et al., 1983). Later, several studies of experimental myocardial I/R showed deposition of complement; C1 (Vakeva et al., 1994), MBL and C3 (Walsh et al., 2005), C4, C5 (McManus et al., 1983; Crawford et al., 1988), C6 (Ito et al., 1996), and C5b-9 (Vakeva et al., 1994). MBL knock-out (KO) mice were protected from heart I/R injury, but when reconstituted with human recombinant MBL, I/R injury similar to that in wild type mice was found. Mice deficient in C2 and factor B (C2/B KO) were also protected form heart I/R injury but no protection was found in C1q or factor D KO mice (Walsh et al., 2005). In a murine model of coronary artery ischemia the involvement of natural IgM antibodies has been linked to both the classical and the lectin pathways (Zhang et al., 2006a; Zhang et al., 2006b). Mice bearing an altered natural IgM repertoire were significantly protected based on the reduced infarct size, limited apoptosis of cardiomyocytes, and decreased neutrophil infiltration.
In addition to direct lytic activity, C5b-9 also directly attenuates endothelium-dependent (i.e., NO-mediated) relaxation (Stahl et al., 1995). Furthermore, C5b-9 is found to play a role in leucocyte activation, adherence and chemotaxis by induction of different cytokines (Kilgore et al., 1996; Vakeva et al., 1998). Also, activation of endothelial NF-kB and the stimulation of expression of endothelial adhesion molecules have been observed. Furthermore, a role for C5b-9 in mediating myocardial apoptosis after ischemia-reperfusion was demonstrated in a model of rat myocardial IRI (Vakeva et al., 1998). We have shown that increased complement activity as indicated by MBL and sC5b-9 levels, was associated with increased risk of cardiac dysfunction in STEMI patients treated with pPCI (Haahr-Pedersen et al., 2009). High plasma MBL and low plasma sC5b-9 was independently associated with increased risk of cardiac dysfunction, likely due to increased complement activity during the ischemic and reperfusion process. The predictive value of low peripheral plasma sC5b-9 may be explained by an accumulation and activation of sC5b-9 in the infarcted myocardium. Furthermore, an elevated plasma sC5b-9 level was found in patients with chronic heart failure due to IHD (Bjerre et al., 2010). In addition, an independent association between sC5b-9, insulin resistance and endothelial dysfunction was found, which may suggest that insulin resistance leading to endothelial activation results in activation of the complement system thus damaging the heart.
Therapeutic inhibition of complement in IHD
Based on the studies discussed above, regulation of the complement system seems to be of great importance. Indeed, several animal studies point to a possible beneficial role of complement depletion in the treatment of post-ischemic MI; reviewed by (Bjerre et al., 2008; Diepenhorst et al., 2009). Unfortunately, clinical studies focused on complement depletion in humans, especially the use of anti-C5 antibodies, primarily Pexelizumab, have largely been disappointing and not proven effective in the setting of CVD, discussed in details elsewhere: (Bjerre et al., 2008; Diepenhorst et al., 2009; Banz and Rieben, 2011).
Although much of the preclinical work and data accumulated in the past years have been positive, the problem with complement-mediated damage in IR-injury was not "easy to fix". Thus conclusions drawn from animal studies should be extrapolated to the human settings with caution. It may lie in the fact that complement activation represents only a part of the cascade of attack directed against the vasculature. An exaggerated blockade of the immune system may increase the susceptibility to infections, thus a well-balanced inhibition of the complement activation is required in order to avoid side effects of pharmaceutically induced modification of the immune system. Direct targeting may be the key to future treatment strategies, including targeting of the complement inhibitor to the site of injury by localized intravascular application.
C-reactive protein
C-reactive protein (CRP), an acute-phase reactant mainly produced by hepatocytes in response to interleukin (IL) 6, is a nonspecific marker of systemic inflammation, and is the most intensively studied inflammatory biomarker in CVD. In addition, a large number of reports have shown that CRP may be implicated in the pathogenesis of many chronic diseases, including diabetes, cancer and alzheimer dementia, and major CVDs, such as, coronary heart disease (CHD), AMI and IHD (Clyne and Olshaker, 1999; Hirschfield and Pepys, 2003; Lavie et al., 2009b). Of note, CRP levels has been associated with the size of the infarct (de Beer et al., 1982).
The concentration of CRP increases rapidly; it may raise hundreds-fold after acute tissue injury or inflammation, and declines rapidly with resolution of the injurious process. In healthy persons, normal CRP levels are generally considered to be < 3 mg/L (Shine et al., 1981).
High sensitive CRP and IHD
In the 1990s, rapid and more precise methods of quantifying CRP was established and CRP levels measured as low as 0.04 mg/L were possible, referred to as high sensitive (hs) CRP (Jaye and Waites, 1997). Low-grade inflammation is found in IHD, and CRP levels are not as increased as compared to other inflammatory conditions. Therefore, a cardiovascular risk scale according to hsCRP levels is recommended by the American Heart Association and Centers for Disease Control and Prevention (Pearson et al., 2003).
Individuals with hsCRP levels;
• <1 mg/L are at lower relative risk for cardiovascular events,
• 1 to 3 mg/L are at intermediate risk,
• >3 mg/L are at higher relative risk.
In the Copenhagen City Heart study, investigating more than 10,000 apparently healthy participants, elevated hsCRP levels were found to be associated with increased risk of IHD. In fact, the risk of IHD was more than doubled (HR=2.2, 1.6-2.9) in individuals with CRP levels above 3 mg/L as compared to individuals with CRP levels below 1 mg/L (Zacho et al., 2008).