Introduction
Coronary artery disease (CAD) is a major cause of death and disability in developed countries. Although CAD mortality rates have declined over the past four decades in the United States (and elsewhere), CAD remains responsible for about one-third of all deaths in individuals over age 35 [1,2]. It has been estimated that nearly one-half of all middle-aged men and one-third of middle-aged women in the United States will develop some manifestation of CAD.
Prevalence
The 2010 Heart Disease and Stroke Statistics update of the American Heart Association reported that 17.6 million persons in the United States have CAD, including 8.5 million with myocardial infarction (MI) and 10.2 million with angina pectoris [2]. The reported prevalence increases with age for both women and men. In a 2009 report that used National Health and Nutrition Examination Survey (NHANES) data, MI prevalence was compared by sex in middle-aged individuals (35 to 54 years) during the 1998 to 1994 and 1999 to 2004 time periods [3]. Although MI prevalence was significantly greater in men than women in both time periods (2.5 versus 0.7 and 2.2 versus 1.0 respectively), there were trends toward a decrease in men and an increase in women. Data from NHANES (and other databases) that rely on self-reported MI and angina from health interviews probably underestimate the actual prevalence of advanced CAD.
This is likely as advanced occlusive coronary artery disease often exists with few symptoms or overt clinical manifestations. Silent ischemia, which is thought to account for 75 percent of all ischemic episodes [4], may be brought to light by electrocardiographic changes (ST segment depression) on an exercise test, ambulatory 24 hour electrocardiographic recording, or periodic routine electrocardiogram (ECG).
Global trends
Heart disease mortality has been declining in the United States and in regions where economies and health care systems are relatively advanced, but the experience is often quite different around the world [5]. Coronary artery disease is the number one cause of death in adults from both low- and middle income countries as well as from high-income countries [5]. At the turn of the century, it was reported that CAD mortality was expected to increase approximately 29 percent in women and 48 percent in men in developed countries between 1990 and 2020. The corresponding estimated increases in developing countries were 120 percent in women and 137 percent in men [6].
The most dramatic increments in ischemic heart disease events on a percentage basis were forecast for the Middle East and Latin America. The experience in Asia is especially important because of the large populations involved. In India, CAD may not be largely explained by traditional risk factors [7]. In China, risk factor trends complement tracking of event rates. For example, the dramatic increase in CAD mortality in Beijing is attributable to greater cholesterol levels. The mean cholesterol level was 4.30 mmol/L (166 mg/ dL) in 1984 and 5.33 mmol/L (206 mg/ dL) only 15 years later [8]. In Latin America, declines in vascular disease rates have been less favorable than in the United States; unfavorable trends in physical activity, obesity, and smoking contribute to these differences [9].
International leaders have called for action plans to avert the projected global epidemic of ischemic heart disease in developing countries [10].
Pathophysiology
Cellular level
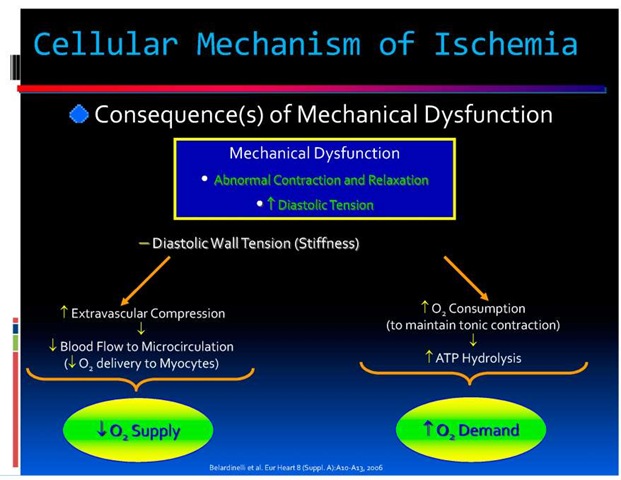
Angina is caused by myocardial ischemia, which occurs whenever myocardial oxygen demand exceeds oxygen supply (Figure 1). Because oxygen delivery to the heart is closely coupled to coronary blood flow, a sudden cessation of regional perfusion following a thrombotic coronary occlusion quickly leads to the cessation of aerobic metabolism, depletion of creatine phosphate, and the onset of anaerobic glycolysis. This is followed by the accumulation of tissue lactate, a progressive reduction in tissue ATP levels, and an accumulation of catabolites, including those of the adenine nucleotide pool. As ischemia continues, tissue acidosis develops and there is an efflux of potassium into the extracellular space. Subsequently, ATP levels fall below those required to maintain critical membrane function, resulting in the onset of myocyte death. Irreversible myocardial injury begins after 20 minutes of coronary occlusion in the absence of significant collaterals [11].
Fig. 1. Cellular Mechanism of Ischemia
Irreversible injury begins in the subendocardium and progresses as a wave front over time, from the subendocardial layers to the subepicardial layers. This reflects the higher oxygen consumption in the subendocardium and the redistribution of collateral flow to the outer layers of the heart by the compressive determinants of flow at reduced coronary pressure. Factors that increase myocardial oxygen consumption (e.g., tachycardia) or reduce oxygen delivery (e.g., anemia, arterial hypotension) accelerate the progression of irreversible injury. In contrast, repetitive reversible ischemia or angina prior to an occlusion can reduce irreversible injury through preconditioning [12].
Anatomical level
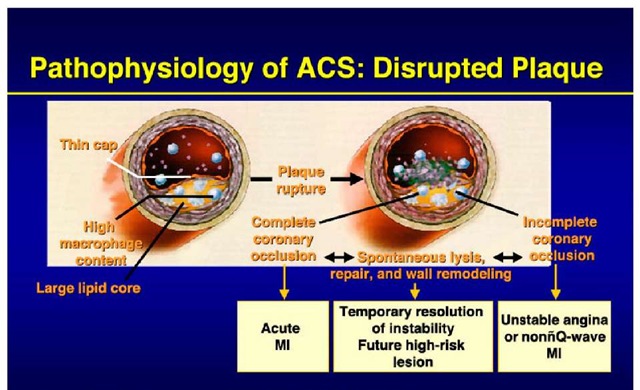
Acute coronary syndrome is usually caused by an unstable atherosclerotic plaque rupture with subsequent platelet-rich thrombus overlying the culprit lesion causing severe narrowing (Figure. 2). This abrupt decrease in blood supply often results in chest pain and ECG changes indicative of ischemia, and, if prolonged, results in myocardial necrosis and enzyme elevation. Less commonly, non ST elevation myocardial infarction is caused by diseases in which myocardial demand exceeds myocardial supply causing a similar clinical presentation. These diseases usually cause a hypermetabolic or high cardiac output state and include hyperthyroidism, anemia, fever, pheochromocytoma, hypertrophic cardiomyopathy, AV fistula and hypertensive urgency/emergency.
Fig. 2. Pathophysiology of ACS:Disrupted Plaque
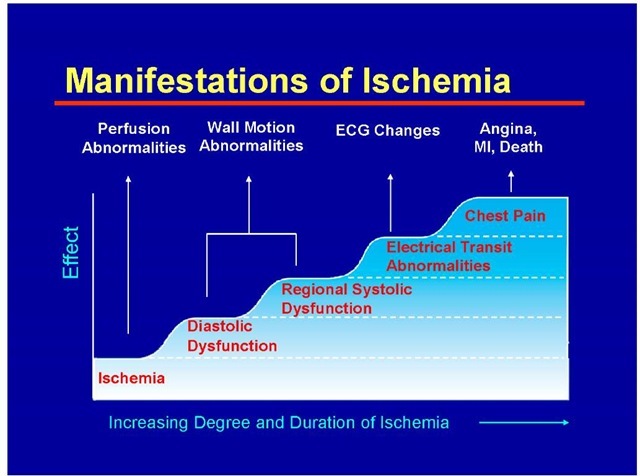
Whatever the mechanism of angina may be, patients usually develop the clinical manifestations in a sequence as described in Figure 3. Note that diastolic dysfunction is the earliest manifestation of ischemia, next to perfusion abnormalities which are seen in nuclear studies.
Clinical features
Coronary artery disease could be manifesting as a continuum from stable angina to acute coronary syndrome. Angina pectoris is a discomfort in the chest or adjacent areas caused by myocardial ischemia. It is usually brought on by exertion and is associated with a disturbance in myocardial function, without myocardial necrosis.
Fig. 3. Manifestations of Ischemia
Heberden’s initial description of angina as conveying a sense of "strangling and anxiety" is still remarkably pertinent. Other adjectives frequently used to describe this distress include viselike, constricting, suffocating, crushing, heavy, and squeezing. In other patients, the quality of the sensation is more vague and described as a mild pressure-like discomfort, an uncomfortable numb sensation, or a burning sensation.
The site of the discomfort is usually retrosternal, but radiation is common and usually occurs down the ulnar surface of the left arm; the right arm and the outer surfaces of both arms may also be involved. Epigastric discomfort alone or in association with chest pressure is not uncommon. Anginal discomfort above the mandible or below the epigastrium is rare. Anginal equivalents (i.e., symptoms of myocardial ischemia other than angina), such as dyspnea, faintness, fatigue, and eructations, are common, particularly in the elderly.
A history of abnormal exertional dyspnea may be an early indicator of CAD even when angina is absent or no evidence of ischemic heart disease can be found on the electrocardiogram (ECG). Dyspnea at rest or with exertion may be a manifestation of severe ischemia, leading to increases in left ventricular (LV) filling pressure. Nocturnal angina should raise the suspicion of sleep apnea.
The term acute coronary syndrome (ACS) is applied to patients in whom there is a suspicion of myocardial ischemia. There are three types of ACS: ST elevation (formerly Q-wave) MI (STEMI), non-ST elevation (formerly non-Q wave) MI (NSTEMI), and unstable angina (UA). The first two are characterized by a typical rise and/or fall in biomarkers of myocyte injury.
For many years, the diagnosis of acute MI relied on the revised criteria established by the World Health Organization (WHO) in 1979. These criteria were epidemiological and aimed at specificity. A joint European Society of Cardiology (ESC) and American College of Cardiology (ACC) committee proposed a more clinically based definition of an acute, evolving, or recent MI in 2000 (13). In 2007 the Joint Task Force of the European Society of Cardiology, American College of Cardiology Foundation, the American Heart Association, and the World Health Federation (ESC/ACCF/AHA/WHF) refined the 2000 criteria and defined acute MI as a clinical event consequent to the death of cardiac myocytes (myocardial necrosis) that is caused by ischemia (as opposed to other etiologies such as myocarditis or trauma) [14].
The criteria used to define MI differ somewhat depending upon the particular clinical circumstance of the patient: those suspected of acute MI based upon their presentation, those undergoing either coronary artery bypass graft surgery or percutaneous intervention, or those who have sustained sudden unexpected, cardiac arrest with or without death [14].
For patients who have undergone recent revascularization or who have sustained cardiac arrest or death, the criteria for the diagnosis of MI are given in detail in the Table 1.
For all other patients in whom there is a suspicion of MI, a typical rise and/or gradual fall (troponin) or more rapid rise and fall (CK-MB) of biochemical markers of myocardial necrosis, with at least one of the following is required:
• Ischemic symptoms
• Development of pathologic Q waves on the ECG
• ECG changes indicative of ischemia (ST segment elevation or depression)
• Imaging evidence of new loss of viable myocardium or a new regional wall motion abnormality.
In addition, pathologic findings (generally at autopsy) of an acute MI are accepted criteria.
The joint task force [14] further refined the definition of MI by developing a clinical classification according to the assumed proximate cause of the myocardial ischemia.
Patulous aneurysmal dilation involving most of the length of a major epicardial coronary artery is present in approximately 1 to 3 percent of patients with obstructive CAD at autopsy or angiography. This angiographic lesion does not appear to affect symptoms, survival, or incidence of MI. Most coronary artery ectasia and/or aneurysms are caused by coronary atherosclerosis (50 percent), and the rest are caused by congenital anomalies and inflammatory diseases, such as Kawasaki disease.
|
Type 1 |
|
Spontaneous myocardial infarction related to ischaemia due to a primary coronary event such as plaque erosion and/or rupture, Assuring, or dissection |
|
Type 2 |
|
Myocardial infarction secondary to ischaemia due to either increased oxygen demand or decreased supply, e.g, coronary artery spasm, coronary embolism, anaemia, arrhythmias, hypertension, or hypotension |
|
Type 3 |
|
Sudden unexpected cardiac death, including cardiac arrest, often with symptoms suggestive of myocardial ischaemia, accompanied by presumably new STelevation, or new LBB6, or evidence of fresh thrombus in a coronary artery by angiography and/or at autopsy, but death occurring before blood samples could be obtained, or at a time before the appearance of cardiac biomarkers in the blood |
|
Type 4a |
|
Myocardial infarction associated with PCI |
|
Type 4b |
|
Myocardial infarction associated with stent thrombosis as documented by angiography or at autopsy |
|
Type 5 |
|
Myocardial infarction associated with CABG |
Table 1. Types of Myocardial Infarction
Diagnosis
Evaluation of new onset chest pain in stable individuals should begin with the consideration of imminently life-threatening causes (including acute coronary syndrome, pulmonary embolus, aortic dissection, pneumothorax, and esophageal rupture). This is usually accomplished using clinical judgement, along with ECG testing, and less frequently exercise testing, other noninvasive testing, or invasive angiography.
This is being discussed in detail by other authors in various topics. nce a life-threatening etiology has been excluded, attempts should be made to identify the specific cause of symptoms and begin treatment. A diagnostic pattern will frequently emerge, based upon the patient’s risk factors, description of the pain, and associated symptoms.
Standard clinical characteristics routinely obtained during the initial medical evaluation of patients with UA/NSTEMI can be used to construct a simple classification system that is predictive of risk for death and cardiac ischemic events. The TIMI (Thrombolysis in Myocardial Infarction) risk score (Table 2) includes variables that can be easily ascertained when a patient with UA/NSTEMI presents to the medical care system. The variables used to construct the score were based on observations from prior studies of risk stratification and incorporate demographic and historical features of the patient, measures of the tempo and acuity of the presenting illness, and indicators of the extent of myocardial ischemia and necrosis.
Since patients with an acute coronary syndrome are at increased risk of death and nonfatal cardiac events, clinicians must assess prognosis on an individual basis to formulate plans for evaluation and treatment. The TIMI risk score for UA/NSTEMI is a simple prognostication