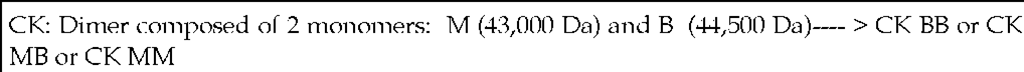Introduction
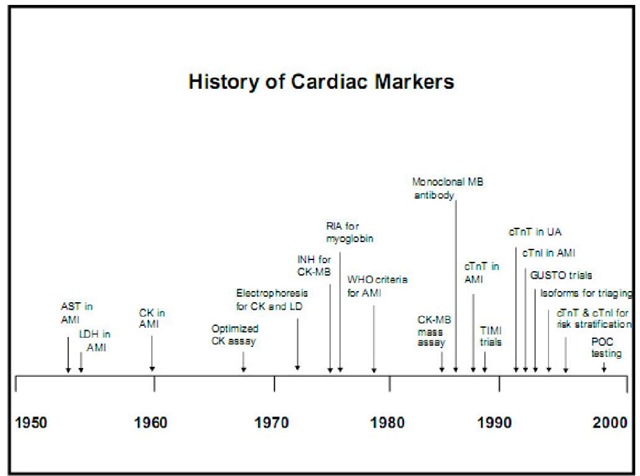
1950′s: Clinical reports that transaminases released from dying myocytes could be detected via laboratory testing, aiding in the diagnosis of myocardial infarction. The race to define clinical markers to aid in the diagnosis, prognosis, and risk stratification of patients with potential cardiovascular disease begins. Initial serum markers included AST, LDH, total CK and a-hydroxybutyrate. These enzymes are all released in varying amounts by dying myocytes. Lack of sensitivity and specificity for cardiac muscle necrosis fuels continued research.
1960′s:CK known to be released during muscle necrosis (including cardiac).Quantitative assays were cumbersome and difficult to perform. Total CK designed as a fast, reproducible spectrophotometric assay in the late 1960′s.CK isoenzymes are subsequently described: MM, MB and BB fractions.
In 1970′s MB fraction noted to be elevated in and highly specific for acute MI.
CKMB now measured via a highly sensitive monoclonal antibody assay. It was felt for a time that quantitative CKMB determination could be used to enzymatically measure the size of an infarct. This has been complicated by release of additional enzymes during reperfusion. As CK-MB assays become more sensitive, researchers come to the paradoxical realization that it too is not totally cardiac specific. The MB fraction is determined to be expressed in skeletal muscle, particularly during the process of muscle regeneration and the search for cardiac specificity continues.
Research turns towards isolation of and development of assays for sarcomeric proteins. Myosin light chains were originally isolated and then subsequently abandoned because of specificity issues. Troponin I first described as a biomarker specific for AMI in 1987; Troponin T in 1989. Now troponins are the biochemical "gold standard" for the diagnosis of acute myocardial infarction via consensus of ESC/ACC.
Fig. 1. Timeline showing landmark events in the development of cardiac biomarkers 1.1 Cardiac Markers: What are we looking at?
A biomarker is defined as a measurable substance or parameter that is an indicator of an underlying biological or pathological process. Therefore, depending on the underlying process that we are referring to, the cardiac markers can be classified as markers of necrosis, markers of ischemia and markers of inflammation. The features of an ideal cardiac marker would be:
• High sensitivity and specificity
• Rise and fall rapidly after ischemia
• Able to perform reliably and uniformly
• Be simple to perform
• Have turnaround time <60 min
• Not influenced by functioning of other organs, in particular, functioning of kidney.
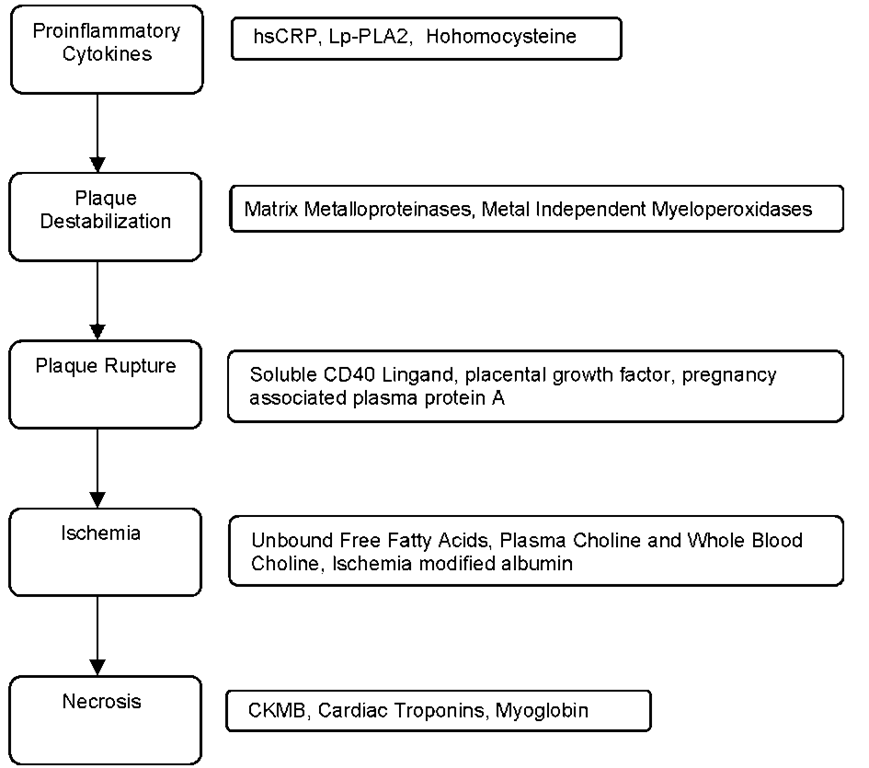
Therefore, cardiac marker is an umbrella term which is used to define present day used necrosis markers as well as all the upstream markers of necrosis studied/under study including proinflammatory cytokines, cellular adhesion molecules, acute phase reactants, plaque destabilization biomarkers, plaque rupture biomarker and prenecrosis ischemia biomarkers. This can be simply visualized with the help of following flow diagram:
Fig. 2. Flowchart showing significance of biomarkers at various levels in the pathogenesis of acute coronary syndrome
Markers of cardiac necrosis
Cardiac markers are used in the diagnosis and risk stratification of patients with chest pain and suspected acute coronary syndrome (ACS). The cardiac troponins, in particular, have become the cardiac markers of choice for patients with ACS. Indeed, cardiac troponin is central to the definition of acute myocardial infarction (MI) in the consensus guidelines from the American College of Cardiology (ACC) and the European Society of Cardiology.
Older Definition of Myocardial Infarction- WHO 1979
1. Definite acute myocardial infarction-Definite acute myocardial infarction is diagnosed in the presence of unequivocal EKG changes and/or unequivocal enzyme changes; the history may be typical or atypical.
2. Possible acute myocardial infarction-Possible acute myocardial infarction is diagnosed when serial, equivocal ECG changes persist more than 24 hours, with or without equivocal enzyme changes; the history may be typical or atypical.
3. Old myocardial infarction-Old myocardial infarction is usually diagnosed on an unequivocal ECG in the absence of a history or enzymatic signs of acute myocardial infarction. If there are no residual ECG changes, the diagnosis may be based on earlier, typical ECGs or on the presence of prior unequivocal serum enzyme changes.
Redefinition of Myocardial Infarction- Joint Task Force of the European Society of Cardiology, American College of Cardiology Foundation, the American Heart Association, and the World Health Federation (ESC/ACCF/AHA/WHF) 2007
A typical rise and/or gradual fall (troponin) or more rapid rise and fall (CK-MB) of biochemical markers of myocardial necrosis, with at least one of the following is required:
• Ischemic symptoms
• Development of pathologic Q waves on the ECG
• ECG changes indicative of ischemia (ST segment elevation or depression)
• Imaging evidence of new loss of viable myocardium or a new regional wall motion abnormality.
In addition, pathologic findings (generally at autopsy) of an acute MI are accepted criteria.
Markers of cardiac necrosis have come a long way since 1950s. Some of the markers used in the past are no longer in use today. Current markers and those used in the past have been outlined in the table below. Those used in the past are not discussed separately further.
|
Current Cardiac Markers |
Cardiac Markers of the Past |
|
• CK-MB |
• Total CK Activity |
|
• Myoglobin |
• Aspartate Aminotransferase Activity |
|
• CKMB Isoforms |
• Lactate Dehydrogenase Activity |
|
• Troponin I and T |
• LD1/LD2 Ratio |
Table 1. Various present day and past cardiac biomarkers
Creatine kinase
The enzyme creatinine kinase (formerly referred to as creatinine phosphokinase) exists as three isoenzyme forms: CK-MM, CK- MB, and CK-BB. These isoenzymes are found in the cytosol and facilitate the egress of high energy phosphates into and out of mitochondria.
Table 2. Isoenzymes of Creatinine Kinase
Distribution of CK: Creatine Kinase (CK) isoenzyme activity is distributed in a number of tissues. The percentage of CK-MB fraction found in the heart is higher than in most other tissues. However, sensitive radioimmunoassays are able to detect small amounts of B chain protein in skeletal muscle, and some muscles have been reported to contain up to 10 percent B chain protein. Most muscles have much more CK per gram than heart tissue .As a result, despite containing only a small percent of B chain protein, skeletal muscle breakdown can lead to absolute increases in CK-MB in the plasma. Therefore, skeletal muscle damage can confound the diagnosis of an MI, as CK-MB can be released. The following are examples:
• Myocardial injury after cardiopulmonary resuscitation
• Cardioversion
• Defibrillation
• Cardiac and non-cardiac surgical procedures
• Blunt chest trauma with possible cardiac contusion
• Cocaine abuse
Total CK, CK-MB and CK-MB to Total CK ratio: Since CK is widely distributed in tissues, elevations in total serum CK lack specificity for cardiac damage, which improves with measurement of the MB fraction. The normal range of CK also varies considerably; a twofold or greater increase in the CK concentration is required for diagnosis. This criterion can be problematic in older individuals who, because of their lower muscle mass, may have low baseline serum total CK and, during MI, may have elevated serum CK-MB with values of total CK that rise but remain within the normal range. For these reasons, total CK has not been used in the diagnosis of myocardial damage for years. CK-MB has high specificity for cardiac tissue and was the preferred marker of cardiac injury for many years. An elevated CK-MB is relatively specific for myocardial injury, particularly in patients with ischemic symptoms when skeletal muscle damage is not present. Assays for CK-MB can be performed easily and rapidly. Most assays measure CK-MB mass; such measurements are more sensitive than activity assays. The relative index calculated by the ratio of CK-MB (mass) to total CK can assist in differentiating false-positive elevations of CK-MB arising from skeletal muscle. A ratio of less than 3 is consistent with a skeletal muscle source, while ratios greater than 5 are indicative of a cardiac source. Ratios between 3 and 5 represent a gray zone. No definitive diagnosis can be established without serial determinations to detect a rise. Studies to evaluate the CK-MB relative index compared with the absolute CK-MB have revealed increase in specificity but with a loss of sensitivity. The CK-MB/CK relative index is useful if patients have only an MI or only skeletal muscle injury, but not if they have both. In the combined setting of acute MI and skeletal muscle injury (rhabdomyolysis, heavy exercise, polymyositis), the fall in sensitivity is significant. It is worth noting that the diagnosis of acute MI must not be based on an elevated relative index alone, because the relative index may be elevated in clinical settings when either the total CK or the CK-MB is within normal limits. The relative index is only clinically useful when both the total CK and the CK-MB levels are increased.
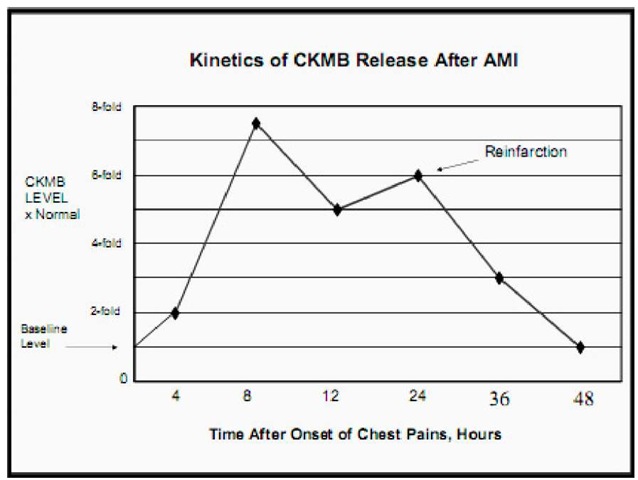
Timing of Release: Creatinine Kinase starts rising in the blood 4-6 hours after the onset of chest pain. It peaks at 10-24 hours and then returns to normal after 48-72 hours. Since CK levels return to baseline 48 to 72 hours after infarction, it can be used to detect reinfarction. New elevations that occur after normalization are indicative of recurrent injury, again with the caveats in regard to sensitivity and specificity indicated above. However, for these reasons, CK-MB cannot be used for late diagnosis.
Fig. 3. Kinetics of CKMB release after AMI
Sensitivity and Specificity of CKMB: In AMI, CKMB usually is evident at 4 to 8 hours, peaks at 15 to 24 hours (mean peak=16*normal) with sensitivity and specificity >97% within the first 48 hours. By 72 hours, two thirds of patients still show some increase in CK-MB. Sampling every 6 hours is more likely to identify a peak value. False negative results may be caused by poor sample timing (e.g. only once in 24 hours or sampling <4 hours or >72 hours after AMI). Similarly, false positive may be caused by a variety of factors including but not limited to myocardial injury after cardiopulmonary resuscitation, cardioversion, defibrillation, cardiac and non-cardiac surgical procedures, blunt chest trauma with possible cardiac contusion and cocaine abuse.
CK and coronary reperfusion :The time to peak CK levels and the slope of CK-MB release can be used to assess whether reperfusion has occurred after fibrinolysis and, when used in conjunction with clinical variables, can predict whether TIMI 0 or 1 and TIMI 2 or 3 grade flow is present. The 2004 task force of the ACC/AHA concluded that serial measurements of CK-MB can be useful to provide supportive noninvasive evidence of reperfusion after fibrinolysis (class IIa recommendation). However, it should be noted that CK-MB criteria cannot identify the presence of TIMI 3 flow, which is the only level of perfusion associated with improved survival after fibrinolysis. Thus, many may elect invasive evaluation despite biomarker evidence of reperfusion. The 2004 ACC/ AHA task force recommended specific guidelines for the diagnosis of reinfarction after an acute ST elevation MI. Within the first 18 hours of the initial MI, a recurrent elevation in CK-MB concentration alone should not be relied upon to diagnose reinfarction, but should be accompanied by recurrent ST segment elevation on ECG and at least one other supporting criterion (such as recurrent chest pain or hemodynamic decompensation). For patients more than 18 hours from the initial MI, a biomarker rise and at least one additional criterion is sufficient for the diagnosis. Similar criteria were established for patients presenting with possible reinfarction after percutaneous coronary intervention or coronary artery bypass grafting.