Diseases of the Basal Ganglia
The circuitry described in the previous sections suggests the presence of a highly sophisticated and delicate set of functional mechanisms that are present within the basal ganglia for the regulation of motor functions. Thus, any disruption of a component of these mechanisms, such as the balance between direct and indirect pathways, will result in significant changes in the signals transmitted to motor regions of the cerebral cortex. Such changes are likely to result in compensatory response mechanisms within the overall circuitry, which will manifest as several kinds of movement disorders. These disorders include involuntary movements during periods of rest (called dys-kinesia), slowness of movement (called bradykinesia), or even a lack of movement (called akinesia). In certain disorders, motor activity is also characterized by hypertonia or rigidity. The following discussion considers the various diseases of the basal ganglia, their relationships with specific neurotransmitter systems, and elements of the circuits within the basal ganglia with which they are likely associated.
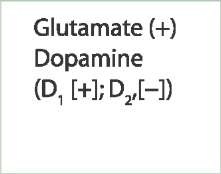
In general, hypokinetic disorders involve impaired initiation of movement, bradykinesia, and increased muscle tone. They are accounted for, in part, by the loss of dopamine inputs into the part of the striatum that
(1) excites the direct pathway through Dt receptors and
(2) inhibits the indirect pathway through D2 receptors. The reduced excitation of the direct pathway (which normally inhibits the internal pallidal segment) results in greater excitation of neurons in the internal pallidal segment, which, in turn, provides further inhibition of tha-lamic neurons and neurons of the motor and premotor areas. This effect is amplified because, at the same time, there is also an increase in excitation of neurons in the external pallidal segment (i.e., indirect pathway) by virtue of reduced inhibition on the GABAergic inhibitory neostriatal projections to this segment (due to the reduction of dopamine on the D2 receptors, whose activation inhibits these neostriatal neurons). The result is an increase in excitation of neurons in the internal pallidal segment because of enhanced release of the excitatory transmitter, glutamate, from the subthalamic nucleus onto this segment of pallidum. These relationships are described in the flow diagram shown in Figure 20-8B.
In contrast, hyperkinetic disorders involve excessive motor activity characterized by marked involuntary movements and decreased muscle tone. These disorders are accounted for by a diminished output through the indirect pathway to the external pallidal segment. Here, a diminished inhibitory input from the neostriatum on the external pallidal segment presumably results from a reduction in the numbers of GABAergic neurons in the neostriatum, which project to the external pallidal segment. This will result in a greater (GABAergic) inhibitory output from the external pallidal segment on the subthalamic nucleus, and the net effect is a reduced excitatory output from the subthalamic nucleus on neurons of the internal pallidal segment. Reduced excitation of the internal pallidal segment will result in reduced inhibition of the thalamus and motor regions of cortex, thus leading to excessive motor activity (Fig. 20-8C).
Parkinson’s Disease
Parkinson’s disease is characterized by a variety of symptoms. The patient displays involuntary movements at rest. The movements are typically rhythmic tremors at approximately 3 to 6 Hz, often appearing as a "pill-rolling" tremor involving the fingers, hands, and arm. Interestingly enough, the tremor disappears when the patient begins a voluntary movement. The patient also displays a reduced number of spontaneous movements (akinesia [e.g., reduction in spontaneous facial expressions and eye blinking]) as well as bradykinesia (i.e., slowness of movement). Postural adjustments are awkward. Patients display slower than normal movements, especially with walking, during which they take short steps in a shuffling (propulsive) manner. Other characteristic symptoms include loss of facial expression (i.e., mask-like face), monotonous speech, and marked increases in muscle tone to both the flexors and extensors of the same limb, producing rigidity.
Parkinson’s disease is now known to result from a loss of the dopamine-containing neurons of the pars compacta of the substantia nigra. The loss of these neurons leads to reduced quantities of dopamine content in the neostria-tum, while other neurotransmitters within the neostria-tum, such as acetylcholine and glutamate, appear to be unaffected. It should be noted, however, that a patient with Parkinson’s disease might also exhibit reduced amounts of norepinephrine and serotonin elsewhere in the brain. Such observations indicate the heterogeneity of this disease with respect to behavioral and neurochemical variations present in different patients.
Because of the known association between reductions in dopamine content within the neostriatum and the onset of symptoms of Parkinson’s disease, several lines of treatment therapies have been developed. One form of treatment involves the administration of the drug, L-3, 4-hydroxyphenylalanine (L-DOPA.L-DOPA passes through the blood-brain barrier, and as a precursor of dopamine, it is converted to dopamine in the brain. Thus, this treatment helps to replenish the reduced amounts of dopamine present in the neostriatum of the patient with Parkinson’s disease. This form of drug therapy has also been supplemented with the drug, carbidopa, which is a dopamine-decarboxylase inhibitor. A drug containing both L-DOPA and carbidopa is called Sinemet. The use of Sinemet has enabled more L-DOPA to reach the neostriatum. Early studies with L-DOPA were shown to be highly successful. However, difficulties have arisen with this form of therapy. Because L-DOPA is administered systemically, it can also result in increased quantities of dopamine in other parts of the brain where dopamine receptors are present. One location is the hypothalamus, which is associated with such functions as feeding, sexual behavior, and control of blood pressure. Consequently, administration of L-DOPA has had such side effects as reducing appetite, causing nausea, heightening sexual interests, and reducing the control of blood pressure. Moreover, because dopamine neurons in the substantia nigra continue to die over time, these forms of drug therapy only work temporarily.
While the etiology of Parkinson’s disease remains unknown, a discovery was made in 1982 that relatively young drug abusers developed signs of Parkinson’s disease after taking synthetic forms of heroin. The drug that was used was toxic and included 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (called MPTP). Because use of MPTP resulted in Parkinson’s disease, it was suggested that it was toxic to dopamine neurons and that environmental toxins may play a role in the development of this disorder. Research has indicated that MPTP has to be converted by monoamine oxidase to 1-methyl-4-phenylpyridinium (MPP+). This is a pyridinium ion. Therefore, it was suggested that blockade of monoamine oxidase would raise dopamine levels and, thus, slow the progress of Parkinson’s disease. Consistent with this view was the observation that a monoamine oxidase inhibitor, L-deprenyl, selectively inhibited monoamine oxidase in the central nervous system and retarded the development of Parkinson’s disease in humans. An important positive outcome of the discovery that MPTP can induce Parkinson’s disease is that it provided investigators with an important experimental tool by which this disorder can be studied in a systematic manner. The use of this toxin as an experimental tool has allowed investigators to confirm the fact that Parkinson’s disease results from loss of dopamine neurons in the pars compacta of the substantia nigra, with a consequent loss of dopamine levels in the neostriatum, and has led to other important findings concerning this disorder.
A second form of possible therapy for Parkinson’s disease is transplantation. This procedure remains in the early stages of experimentation. Here, embryonic tissue containing dopamine neurons are transplanted directly into the neostriatum. These dopamine-containing cells then grow sprouts, synthesize, and release dopamine onto neos-triatal neurons, where they serve to increase dopamine levels. Embryonic cells (in suspension) are used because there appears to be little or no evidence of rejection from such tissue. While this procedure has met with some preliminary success, difficulties remain. In particular, it is not possible to implant embryonic cells in all regions of the neostriatum. Because it is not clear which regions of the neostriatum are most critical for replenishment of dopamine levels, the effectiveness of this approach remains questionable. Another major problem is the difficulty in obtaining sufficient quantities of embryonic tissue to carry out the required procedures. The inherent legal and ethical issues remain formidable ones.
A related developing strategy is to graft stem cells into regions where neurons have undergone degeneration as a result of a disease process. Stem cells constitute the master cells of the organism that are capable of differentiating into different cell types. There are two types of stem cells: embryonic and adult. It has been argued that embryonic cells have several advantages. They can be obtained in large numbers and have the capacity of becoming all cell types that can easily be grown in culture. These features are not as clearly evident with adult stem cells. Nevertheless, the hope is that stem cells, whether embryonic or adult, can be implanted into the appropriate brain loci and that these cells can be differentiated into dopamine neurons. In this way, this approach may serve as a method for replenishment of dopamine in the regions affected by the destruction of dopamine neurons in the substantia nigra. Recent studies using this approach in animals have provided promising data.
At least three other approaches have been used. For reasons that we have just indicated, a reduced dopaminer-gic input to the neostriatum results in a hypokinetic disorder of which Parkinson’s disease is a prime example. There are many cholinergic neurons in the neostriatum, which are excitatory to neostriatal projection neurons that supply the external pallidal segment (indirect pathway). Therefore, it is possible that the hypokinetic disorder occurs in part because of the actions of these cholinergic neurons, whose effects become more pronounced by the reduced inhibitory actions of dopamine acting through D2 inhibitory receptors in the neostriatum (Fig. 20-8B). This line of reasoning represents the rationale for an approach that involves the use of anticholinergic drugs to reduce the imbalance between dopamine and acetylcholine (Ach) levels in the neostriatum. This treatment results in a reduction in the excitatory effects generated by Ach and has met with some success in patients.
Alternatively, some success has been reported in patients subjected to thalamotomy involving the ventro-lateral and, perhaps, the ventral anterior nuclei. However, the rationale for this approach, which is to reduce the aberrant signals transmitted to the motor regions of the cortex from the basal ganglia, is conceptually difficult to understand because there already is a reduced output of the thalamocortical projections in Parkinson’s disease. Other problems exist with this procedure such as the difficulty in making accurate lesions in the appropriate region of the thalamus that eliminate symptoms of the disorder. Moreover, the placement of such lesions is also questionable because it is not known with any certainty which regions are the critical ones.
Another therapeutic approach has been electrical stimulation to parts of the basal ganglia and related structures, such as the pallidum, subthalamic nucleus, and ventral anterior and ventral lateral nuclei of thalamus. In this procedure, a stimulating electrode is implanted into one of these structures, and, when current is passed through the tip of the electrode, involuntary movements are suppressed. This method is quite new, so the overall effectiveness and pitfalls of this approach have yet to be adequately determined.
Chorea (Huntington’s Disease)
In general, chorea is characterized by wild, uncontrolled movements of the distal musculature, which appear as abrupt and jerky. Huntington’s disease is an inherited autosomal dominant illness with the genetic defect located on the short arm of chromosome 4. The gene encodes a protein referred to as huntingtin. In the mutated form, it includes a much longer patch (than normal protein) of glutamine residues. Specifically, the DNA segment (CAG) that encodes glutamine is repeated more than 60 times in the mutated gene as opposed to approximately 20 repeats in the normal gene. Although it is not clear how the mutant gene causes cell death, one hypothesis is that the hunting-tin protein causes an induction of apoptosis in the nucleus of the cell. Perhaps this occurs by the alteration of protein folding due to the increased amounts of glutamines, causing dysfunction and ultimately the death of the cell.
Degeneration is quite extensive. It involves the neos-triatum, where there is significant loss of GABA. As the disease becomes more progressive, it also involves the cerebral cortex and, in particular, the frontal and prefrontal regions, as well as a number of other structures. Damage to these regions causes not only motor damage, but also loss of intellectual functions. The disease is progressive with an onset in the fifth and sixth decades of life. There is also a juvenile form of the disease, because of which patients usually die before the age of 21 years.
The manifestation of the hyperkinetic motor effects inherent in Huntington’s disease may be understood in terms of the effects of the loss of GABAergic neurons in the neostriatum on the indirect pathway. It is believed that there is a preferential loss of GABAergic neurons that project to the external pallidal segment compared with the GABAergic neurons that project elsewhere. As a result of the loss of inhibitory GABAergic inputs to the external pallidal segment from the neostriatum, this segment of the pallidum can now generate greater inhibition on the subthalamic nucleus. This will lead to a consequent loss of excitatory input from the subthalamic nucleus onto the internal pallidal segment. Because the internal palli-dal segment normally inhibits the thalamic projection nuclei to the motor regions of the cortex, loss of subtha-lamic excitatory input to the internal pallidal segment will result in a consequent reduced inhibitory input onto these thalamic nuclei (Fig. 20-8C). This would ultimately result in excess excitation of the motor regions of the cortex, causing the choreiform movements seen in Huntington’s disease.
Hemiballism
Violent involuntary ballistic movements of the limbs contralateral to the lesion characterize hemiballism. The proximal musculature is typically involved, but choreiform (irregular, jerky) movements of the distal musculature usually in the upper extremity (which may also involve the lower extremity) may also be present. In hemiballism, the lesion has been found to be discrete and localized within the subthalamic nucleus, perhaps as a result of a discrete stroke. Since activation of the sub-thalamic nucleus excites inhibitory neurons from the internal pallidal segment that project to the thalamus, lesions of the subthalamic nucleus would cause a significant reduction of these inhibitory influences on the tha-lamus. Thus, this sequence will result in an increase in movement of the proximal and distal musculature on the side of the body opposite the lesion. (Recall that the corticospinal tract is crossed; therefore, activation of one side of the cortex will result in movement of the contralateral side of the body.)
Athetosis and Dystonia
Athetosis is a variant of choreiform movement. It involves slow, writhing movements of the extremities. Dystonia is characterized by sustained muscle contractions of the limb, axial, or cranial voluntary muscles, resulting in abnormal postures and repetitive or twisting movements. If the movements produce severe torsion of the neck or shoulder girdle, causing an appearance of a rhythmic shaking of the head, this form of dystonia is referred to as torsion tremor. Both athetosis and dystonia are believed to be associated with damage to the neostriatum and, possibly, the cerebral cortex.
Tardive Dyskinesia
Involuntary movements of the tongue and face characterize tardive dyskinesia. There are repetitive chewing movements, and the tongue irregularly moves in and out of the mouth. It is not induced by lesions but, instead, is induced by the administration of antipsychotic drugs, such as haloperidol (Haldol), that block dopaminergic transmission. It is believed that such blockade results in dopamine D2 receptor hypersensitivity. Because these dopamine receptors within the neostriatum become more sensitive, dopaminergic transmission to the neostriatum becomes more potent, leading to an alteration in the balanced relationship between dopaminergic, cholinergic, and GABAergic neuronal systems. However, more recent research has suggested that this disorder may be more closely associated with depletion in GABA and its synthesizing enzyme, glutamic acid decarboxylase, in the neostriatum after treatment with antipsychotic drugs. Such compensatory changes produce movements characterized as tardive dyskinesia. Several of the treatments for this disorder are linked to the presumed relationship between dopamine and tardive dyskinesia. These include tapering down of the antipsychotic drug, administering dopamine-depleting drugs, or administering clozapine, which interferes with binding of dopamine receptors. However, after prolonged use of haloperidol, the resulting tardive dyskinesia does not disappear after cessation of drug use.
Tourette Syndrome
The primary features of this disorder are motor and vocal tics. Characteristics of motor tics include brief, sudden involuntary movements of different parts of the body (e.g., shrugging of the shoulder, turning in a circle, and eye-blinking). Vocal tics may involve sounds that are irritating in pitch, compulsive expression of obscenities (called coprolalia), repetition of words (palilalia), and repeating words of others. In many patients, the disorder subsides in early adulthood. In others, the disorder may subside but reappear again at a later period and persist throughout life.
Evidence supports the possibilitiy of a genetic basis for this disorder but knowledge of causal factors remain unknown. Haloperidol, which blocks dopamine D2 receptors, has been shown to be an effective treatment for Tourette syndrome whereas symptoms worsen following L-DOPA administration. Noradrenergic alpha2 receptor agonists (i.e., clonidine and guanfacine) have also been used for treatment of this disorder. It has been suggested that Tourette syndrome is associated with dysfunctions of the caudate nucleus and its linkage with the prefrontal cortex, although anatomical and clinical evidence in support of this view remains incomplete.
Clinical Case
History
Peter is a 67-year-old man who has been having difficulty moving for the past 10 years. His family noticed that he had a slow shuffling gait and had difficulty getting out of a chair. Once he was able to get out of a chair, after an initial slow gait, his gait would become progressively faster. Although in the past he had been a jovial, expressive man, his face appeared to be masked, with very few eye blinks and with little spontaneous emotional reactions. He spoke with a slow monotonous voice, which often became softer as he spoke. After having several fainting spells, his family brought him to a neurologist.
Examination
During the neurological examination, it was noted that Peter’s limbs were stiff, and he exhibited a slow, pill-rolling tremor. When he attempted to write, his handwriting started out as small and became progressively smaller. His family told the neurologist that they thought that he was forgetting progressively more appointments, and they felt that he might be depressed.They also indicated that Peter’s sleep had become increasingly disrupted and that he exhibited leg movements at night.
Explanation
Peter is displaying symptoms of Parkinson’s disease, a degenerative disease caused by a progressive loss of dopaminergic cells from the substantia nigra.The substantia nigra is a component of the basal ganglia, whose functions provide refinement and feedback to cortical motor systems.The net effect of this system results in movements that are smooth and whose goals are reached efficiently. One manifestation of dysfunction of the substantia nigra includes "cogwheel rigidity," in which there are catches during passive movement of a rigid limb. Another classic manifestation is a pill-rolling tremor of the hand at a rate of approximately 3 to 6 Hz, which is slower than most other tremors. Patients with Parkinson’s disease have difficulty when initiating movement, especially in gait and rising from a chair. Spontaneous facial expression is markedly diminished, and masked faces with paucity of movements are a hallmark of Parkinson’s disease. Handwriting is often affected; it may begin with near-normal amplitude, but as the writing progresses, it becomes smaller and smaller, which is a phenomenon called micrographia.
SUMMARY TABLE
Input-Output Relationships of Structures Comprising the Basal Ganglia
GABA = gamma aminobutyric acid; (+) = excitatory; (-) = inhibitory.