Early Aspects of Development
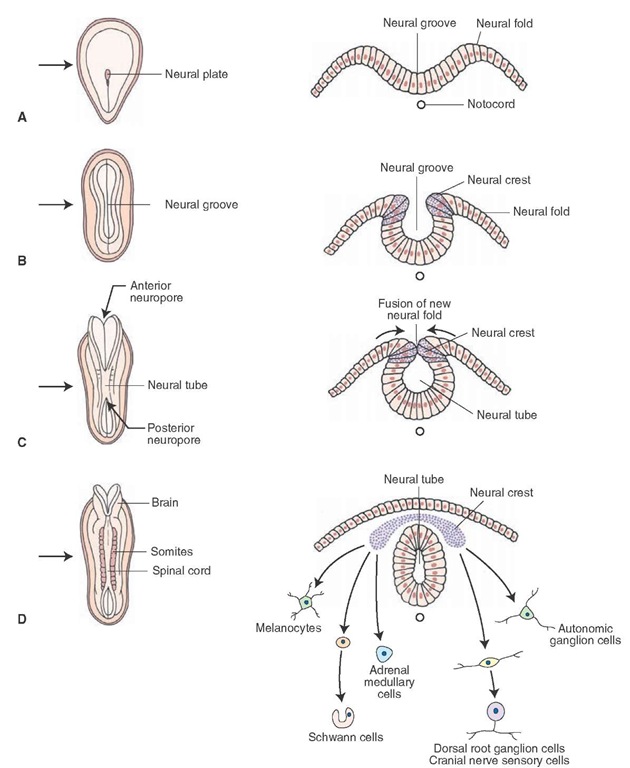
The nervous system develops from ectoderm, the surface layer of embryonic tissue. By the third to fourth week of embryonic development, the notochord, of mesodermal origin, induces the development of the neural plate (Fig. 2-1A). By the third to fourth week of embryonic development, there is a high rate of cell proliferation. As such, the anterior part of the notochord (of mesodermal origin) begins to thicken, and, thus, the neural plate is formed by the third week of fetal life (Fig. 2-1A). The neural plate continues to thicken over the following week and expands laterally. As it expands, the faster growing lateral edges of the plate accumulate in a dorsal position as neural folds (Fig. 2-1B). As this plate grows and widens, it forms a shallow groove along its longitudinal axis known as the neural groove (Fig. 2-1B). The posterior end of the neural plate, which is narrower than the anterior end, will ultimately become the spinal cord, whereas the broader, anterior end will become the brain. As this plate grows and widens, the neural groove becomes deeper. In the process of its forming and deepening, some of the cells located in the lateral margin of the neural groove separate and migrate to a dorsal position to become the neural crest (Fig. 2-1B). As the embryo grows, the neural folds fuse along the midline, thus forming a neural tube (Fig. 2-1C).
FIGURE 2-1 Early embryonic development of the central nervous system. Panels A-D depict early development (at the third and fourth weeks of gestation) in which the neural plate (A), neural groove (B), and neural tube (C) are formed from the dorsal surface of the embryo. The left side of each panel depicts the developing embryo in a dorsal view, and the right side shows cross sections through the nervous system cut at the levels indicated by the arrows. Note also the cells formed from differentiated cells of the neural crest (D).
Neural crest cells will differentiate into separate groups of neurons (Fig. 2-1D). One group differentiates into sensory neurons of cranial nerve (CN) ganglia (components of CN V, VII, IX, and X of the head region) and into the dorsal root ganglia (components of the body). A second group will differentiate into the autonomic ganglion cells (postganglionic neurons of the paravertebral and prever-tebral ganglia of the sympathetic nervous system as well as postganglionic neurons of the parasympathetic nervous system that are located in visceral organs.Other neural crest cells will become chromaffin cells (of the adrenal medulla), Schwann cells (that are critical for the formation of myelin in peripheral nerves), and melanocytes. In addition, groups of mesodermal cells located alongside the neural tube, called somites, will develop into skeletal muscle, vertebrae, and the dermal layer of the skin (Fig. 2-1D).
The anterior aspect of the neural plate develops subdivisions, which will initially form three brain vesicles and, ultimately, five brain vesicles. The three brain vesicles include the prosencephalon, mesencephalon, and rhombencephalon; caudal to these vesicles are cells from which the spinal cord will develop (Fig. 2-2A). The five vesicles derived from these vesicles are as follows: the rostral prosencephalon (forebrain), which will later become the telencephalon and diencephalon, including the cells that will develop into the retina; the mesen-cephalon, which will form the midbrain; and the caudal rhombencephalon (hindbrain), which will later form the metencephalon (pons) and myelencephalon (medulla) (Fig. 2-2B). When depicted in a lateral view, the flexures associated with each vesicle can be seen (Fig. 2-2C).
FIGURE 2-2 Formation of the vesicles of the brain. The figures depict the division of the forebrain vesicle into three (A) and five (B) vesicle stages to form the telencephalon and diencephalon (from the prosencephalon) as well as the formation of the midbrain and the metencephalon and myelencephalon (from the hindbrain). These illustrations are dorsal views of the neural tube at the two different stages in which the flexures are not shown. Panel C depicts these stages in a lateral view. The flexures result from the proliferation of cells in the cerebral hemispheres and brainstem in the cranium, which is somewhat restricted in size.
Morphogenesis of the Central Nervous System
The Spinal Cord
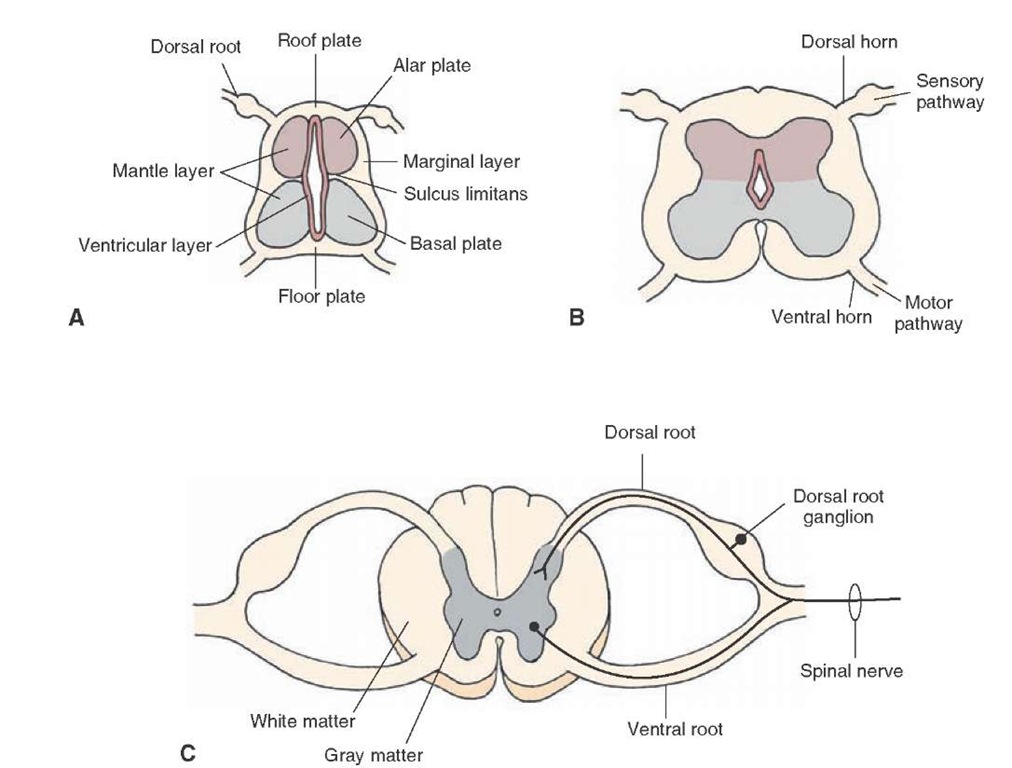
The neural tube (spinal cord) consists of three layers: an inner layer called the ventricular layer, which is in contact with the cavity of the neural tube; an intermediate layer called the mantle layer; and an outer layer called the marginal layer (Fig. 2-3A). The ventricular zone is the major proliferative layer and also the first layer of the forming neural tube to appear. The second layer to form is the marginal layer, followed by the mantle layer. Early in development, the wall of the neural canal becomes thickened, in part, by the formation of young or immature neurons that have yet to completely differentiated (sometimes called neurocytes) in the mantle layer. Because this layer contains the primary cell bodies of neurons, it will ultimately become the gray matter of the spinal cord. Axons associated with cells in the mantle layer will grow into the marginal layer.
The neural tube undergoes additional differentiation that can best be viewed in neural tube cross sections. During proliferation of immature neural cells, a pair of grooves appears at approximately the midpoints along the lateral margin of the neural canal. This groove, the sulcus limi-tans (Fig. 2-3A), is an important anatomical landmark with respect to later development of functional regions of the central nervous system (CNS). In essence, neural cells (neurocytes) accumulate in several locations in relation to the sulcus limitans. Neurocytes migrating dorsal to the sulcus limitans form the alar plate, whereas those migrating ventral to the sulcus limitans form the basal plate (Fig. 2-3A). Moreover, the developing cells that lie in an intermediate position adjacent to the sulcus limitans will become autonomic neurons. Thus, the alar and basal plates form the walls of the neural canal. The cells that lie along the dorsal midline are referred to as the roof plate, and cells that lie along the midline of the ventral aspect of the neural canal are known as the floor plate (Fig. 2-3A). The cells in the alar plate and the basal plate will contribute to sensory pathways and motor pathways, respectively (Fig. 2-3B).
The direction in which axons in the neural tube travel depends on the specific location of these cells within the mantle layer. Axons generally situated more ventrally within the mantle layer (i.e., the basal plate) will invade adjacent segments (called somites) that will constitute different regions of the body. They will become functionally linked by nerve fibers from the mantle layer that will form the ventral root of the spinal cord (Fig. 2-3C). In so doing, these axons will develop into the motor neurons of the nervous system. Axons are initially found in the marginal layer growing toward rostral or caudal levels of the spinal cord or toward the brain. Some of the axons arise from spinal cord neuronal cell bodies in the gray matter; some arise from dorsal root ganglion cells, whose axons form the dorsal root; and, a little later, some will descend from the brain (Fig. 2-3C). Axons added rapidly during development become the characteristic outer white matter of the spinal cord (Fig. 2-3C). The developing spinal nerves contain the following functional components: general somatic afferent (GSA), general somatic efferent (GSE), general visceral efferent (GVE), and general visceral afferent (GVA) neurons. GSA neurons include those that transmit sensory impulses from the periphery to the brain. They transmit such information as changes in temperature, noxious stimulation, touch, pressure, and information involving proprioceptors (i.e., stretch of a muscle, tendon, or bodily position). GSE fibers transmit signals from the CNS to skeletal muscle. GVE fibers, which originate close to the sulcus limitans, transmit autonomic signals from the CNS to smooth muscle and glands. GVA fibers originate from visceral structures and provide the CNS with information concerning their status.
TABLE 2-1 Differentiation of the Neural Tube During Its Development
|
Embryonic Derivative |
Spinal Cord |
Rhombencephalon (Hindbrain), Myelencephalon (Medulla), and Metencephalon (Pons and Cerebellum) |
Mesencephalon (Midbrain) |
Prosencephalon (Forebrain) (Diencephalon and Telencephalon) |
|
Roof plate |
Region of posterior median septum |
Superior medullary velum |
Commissures of the superior and inferior colliculi |
Choroid tela and choroid plexus of the lateral and third ventricles |
|
Alar plate |
Dorsal gray columns |
Sensory nuclei of CN V, VII, VIII, IX, X; cerebellum, deep pontine nuclei, inferior olivary nucleus, mesencephalic nucleus (CN V [but displaced to midbrain]) |
Superior and inferior colliculi, red nucleus, substantia nigra, main sensory nucleus (CN V); some nuclei of reticular formation? |
It has been suggested that diencephalon (thalamus and hypothalamus) telencephalic structures are derived from alar plate, but derivation is still unclear at this time |
|
Basal plate |
Ventral gray columns; nucleus of CN XI |
Motor nuclei of CN V, VI, VII, IX, X, XII; nuclei of reticular formation |
Motor nuclei of CN III, IV; nuclei of reticular formation |
— |
|
Floor plate |
Region of ventral median fissure |
— |
— |
— |
FIGURE 2-3 Development of the spinal cord: (A) early stage of development; (B) intermediate stage of development; and (C) late stage of development. Note also the positions of the alar, basal, and roof and floor plates as shown in panel A and the structures derived from them shown in panels B and C.
An important feature in the development of the spinal cord relates to the relative differences in the rates of growth of the spinal cord in comparison to the vertebral column. Although the growth rates for both during the first 3 months are approximately equivalent, there is a change in the succeeding 4 months. Specifically, during this latter period, the growth rate of the vertebral column is considerably more rapid than that of the spinal cord. Because of the differential rates of growth, at birth, the spinal cord does not fill the entire extent of the neural canal but, instead, reaches only as far as the third lumbar vertebra; the spinal cord reaches the second lumbar vertebra in the adult. This differential rate of growth also alters the orientation of nerve fibers that exit from the spinal cord. Whereas nerve fibers that arise from more rostral levels of the spinal cord exit at approximately right angles, those exiting from more caudal levels become elongated and are oriented much more ventrally.