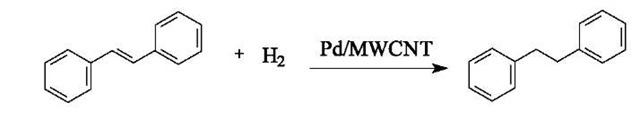Functionalized MWCNTs Decorated with Catalytic Rhodium Nanoparticles
Rh(acac)2xH2O (acac=acetylacetonate) was used as the metal precursor in the hydrogen reduction reaction for loading Rh nanoparticles onto functionalized MWCNTs. The adopted temperature for the reduction was 250°C. A representative TEM image of MWCNTs decorated with Rh nanoparticles is displayed in Fig. 5A. A high and homogeneous dispersion of nanoparticles with a uniform distribution of particle sizes centered around 3-5 nm can be distinguished. The nanoparticles are comparable in size to the diameter of MWCNTs and are crystalline in nature as indicated by the SAED pattern given as an inset in Fig. 5A. The HRTEM image shown in Fig. 5B verifies that Rh nanoparticles are crystallites with visible lattice fringes.
Functionalized MWCNTs Decorated with Catalytic Ruthenium Nanoparticles
Decoration of Ru nanoparticles onto functionalized MWCNTs was performed by using Ru(acac)3 • xH2O as the metal precursor for hydrogen reduction at 250°C. Fig. 6 presents the TEM images of highly dispersed Ru nanoparticles attached on MWCNTs, with very tiny diameters around 1 nm and a uniform distribution throughout the full length of MWCNTs. The SAED pattern exhibits a set of diffraction rings from Ru metal. The diffraction did not appear as clear spots, but as concentric rings, each of which consists of a large number of very small spots, suggesting that the nanoparticles are composed of many fine crystallites. EDS spectrum of the nanocomposite shows emission from Ru, indicating the chemical identity of nanoparticles as Ru containing.
Chemical States of Metal Nanoparticles Decorated onto Functionalized MWCNTs
The chemical composition of the nanoparticles deposited onto functionalized MWCNTs was analyzed by XPS.
Fig. 6 TEM image of highly dispersed Ru nanoparticles attached on MWCNTs.
Survey XPS spectra of the decorated MWCNTs provide results similar to those from EDS. In addition to peaks of C, O, and Si resulting from the MWCNTs and background (MWCNTs were dispersed onto silicon substrates for XPS analysis), each survey XPS spectrum shows strong peaks of Pd, Rh, or Ru. No other heterolement including fluorine was detected, implying no byproducts or unreacted precursors are present in the nanocomposites.
The states of MWCNT-supported palladium, rhodium, and ruthenium nanoparticles can be more clearly identified through high-resolution XPS analysis. A typical high-resolution Pd3d XPS spectrum of the Pd-decorated MWCNTs is shown in Fig. 7A. The binding energies, 335.3 eV for Pd3d5/2 peak and 340.6 eV for Pd3d3/2 peak, are in accordance with those reported for Pd0.[69] Furthermore, the peaks are asymmetric, having a line shape typical of metallic Pd.[69] All these imply that the palladium in the nanoparticles is zero-valent. No significant changes were observed in the binding energies or intensities of the Pd3d XPS core levels after exposing the palladium-decorated MWCNTs to air for 1 month, which demonstrates the stability of the palladium nanoparticles.
Fig. 7B displays the high-resolution Rh3d core level XPS spectrum of MWCNTs coated with Rh nanopaticles. The spectrum shows a low-energy band Rh3d5/2 at 307.3 eV, and a high-energy band Rh3d3/2 centering at 312.1 eV. As the Rh3d5/2 and Rh3d3/2 peaks for rhodium metal lie at 307.2 and 312.0 eV, respectively,1-69-1 this indicates that Rh is also in the zero-valent state in Rh nanoparticle-MWCNT composites.
For MWCNTs decorated with ruthenium nanoparti-cles, the high-resolution Ru3d XPS spectrum has been obscured by the C1s spectrum. The deconvoluted spectrum shown in Fig. 7C gives broad bands that can be curve-fitted into two pairs of Ru3d peaks, therefore two chemically different Ru entities can be identified: the dominant pair with a Ru3d5/2 peak at 279.8 eV corresponds well with the 3d5/2 and 3d3/2 lines of element Ru0, indicating that the majority of ruthenium loaded on MWCNTs is present as metallic Ru; the minor pair showing a Ru3d5/2 peak at 280.8 eV can be assigned to Ru oxides. The presence of Ru oxides is responsible for the strong interaction between highly dispersed Ru nanoparticles and oxygen-containing groups on the functionalized MWCNTs and could also be resulted from the slight oxidation of Ru nanoparticles upon exposure of samples to ambient air.
Fig. 7 High-resolution (A) Pd3d, (B) Rh3d, and (C) Ru3d XPS spectra of MWCNTs decorated with Pd, Rh, or Ru nanoparticles.
Promising Applications of the Metal-MWCNT Nanocomposites in Catalysis
Chemistry in ecologically benign solvents is of increasing interest in recent years. Most solvents used in organic syntheses for heterogeneous or homogeneous catalysis are coming under close scrutiny because of their toxicity and waste generation. There is a great push in industry today to replace these solvents with environmentally friendly solvents such as liquid or supercritical CO2. We have recently reported that palladium nanoparticles dispersed by a water-in-CO2 microemulsion are very effective catalysts for hydrogenation of a number of olefins.[70] However, separation of products from the surfactants and reuse of the catalyst are potential technical difficulties associated with the microemulsion technique. Developing effective heterogeneous catalysts that can be reused for chemical synthesis in liquid or supercritical CO2 is currently of great interest to the chemical industry. The Pd nanoparticle-MWCNT composite may provide an effective catalyst for chemical synthesis in a green solvent that allows easy separation of products and minimizes waste solvent generation.
The catalytic capability of the Pd-MWCNT composite was tested for hydrogenation of a CO2-soluble olefin frans-stilbene in liquid CO2:
In this test, stilbene was dissolved in a mixture of 5 atm H2 and 100 atm of CO2 to make a 0.033 mol/L solution. The solution was pumped into a 6.94-mL stainless steel vessel loaded with 5 mg of the Pd-MWCNT composite (reported in Fig. 4C) at room temperature (23°C). Ultrasonication was applied to the vessel for 10 sec to disperse the catalyst. The product was trapped in CDCl3 at different times and analyzed by proton NMR (Bruker, AMX 300). According to our NMR results, conversion of stilbene to 1,2-diphenylethane was about 80% and 96% after 5 and 10 min of reaction, respectively.
A number of noble metal-carbon fiber or metal porphyrin-graphite composites have been demonstrated to catalyze electrochemical reactions significantly.[13,71,72]
The Pd-MWCNT nanocomposite was also tested for its electrocatalytic activity in oxygen reduction that is important in fuel cell applications. For comparison, graphite powder, MWCNTs, and Pd-MWCNT (Fig. 4C) were mixed individually with mineral oil to make three different carbon paste working electrodes. Cyclic voltam-metry measurements were conducted at room temperature in a three-compartment electrochemical cell. The electrolyte was 1.0 M H2SO4 saturated with oxygen. The potential was cycled between +0.60 and — 0.10 V at 40 mV/sec. As shown in Fig. 8, essentially no O2 reduction was observed over the potential window for the carbon paste electrodes of bare MWCNT and graphite powder. In contrast, for the Pd-MWCNT electrode, a very large O2 reduction wave was observed at potentials characteristic for Pd electrocatalysis. The enhancement of the cathodic current indicates a high electrocatalytic activity of the Pd-MWCNT electrode for the reduction of oxygen.
The highly dispersed Rh and Ru nanoparticles on MWCNTs are also expected to be potential catalysts for a variety of reactions.[26,73] As a catalyst for the hydroge-nation of frans-cinnamaldehyde and the hydroformylation of hex-1-ene in the liquid phase, Rh-supported MWCNTs were found to be very selective toward C=C double-bond hydrogenation and the production of linear and branched aldehydes, respectively.[73] However, Ru nanoparticles anchoring on MWCNTs showed an unexpected increase in selectivity (up to 92%) for cinnamyl alcohol in liquid-phase hydrogenation of cinnamaldehyde.[26]
Fig. 8 Cyclic voltammograms of oxygen reduction in 1.0 M H2SO4.
Fig. 9 SEM images of SiO2 NWs after copper deposition from supercritical CO2 solutions of (A) 2.0 x 10" 3 (B) 2.7 x 10" 2 mol/L Cu(hfa)2.
METAL-NW NANOCOMPOSITES
Metal-SiO2 NW Composites
Modification of SiO2 NWs was performed by hydrogen reduction of Cu(hfa)2 • xH2O in scCO2 at 250°C and 80°C, respectively. Typical SEM images of SiO2 NWs after copper deposition are shown in Fig. 9, and three types of nanostructures are clearly visible. When a lower precursor concentration (2.0 x 10"3 mol/L) was used in the experiment, discrete Cu nanoparticles were found to randomly anchor to the SiO2 NWs (inset of Fig. 9A). As the concentration of the precursor increased to 2.7 x 10"2 mol/L, the NWs became thicker after coating, wrapped by shells of densely packed Cu nanoparticles. The average outside diameter of the wire-shell composite structures can be up to 400 nm, indicating that the shells were composed of multilayers of Cu nanoparticles. The rough surfaces of the composites suggested that the copper coating was polycrystalline. We believe that the Cu nanoparticles were nucleated in the SCF medium and then deposited onto the SiO2 NW surfaces to form the coated layer. Noticeable from the SEM images is also the aggregation of the Cu nanocrystals to form larger structures, most of which were sphere-like and ”strung” up by the NWs. The inset in Fig. 9A shows a TEM image of a nanoparticle-decorated NW. Cu nanoparticles with different sizes, ranging from several to 50 nm in diameter, strung onto the SiO2 NWs.
Metal Alloy-SiC NW Composites
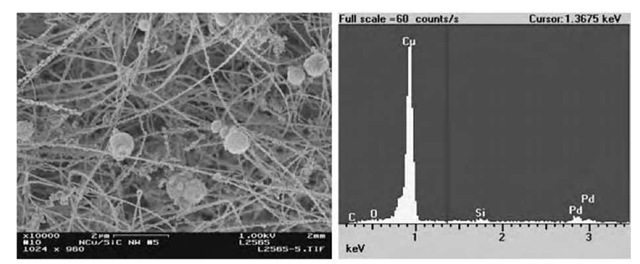
In principle, a number of metal precursors can be used as starting materials as long as they are soluble in CO2, and metals other than palladium and copper, or metal alloys can be coated on the SiO2 NWs to form nanocomposites. Furthermore, NWs suitable for the SCF fabrication process are not limited to SiO2. Such hydrogen reduction of metal precursors in supercritical CO2 may provide a general and clean process to modify NWs with metallic nanoparticles. Fig. 10 describes Cu-Pd alloy nanoparticles attached to SiC NWs through hydrogen reduction of a mixture of Cu(hfa)2 • xH2O (95%) and Pd(hfa)2 • xH2O (5%) in scCO2 at 80°C. Some aggregated balls of metals are also observed.
Fig. 10 Cu-Pd-SiC nanocomposites. (a) SEM image of Cu-Pd nanocrystals supported by SiC NWs, (b) EDS of the nanocomposites.
CONCLUSION
SCF approach is a novel and emerging technology to generate nanomaterials in small areas, high-aspect-ratio structures, complicated surfaces, and poorly wettable substrates with high uniformity, high homogeneity, and minimum environmental problems.
Through hydrogen reduction of metal-p-diketone complexes in supercritical CO2, a rapid, convenient, and environmentally benign approach has been developed to synthesize a variety of nanostructured materials: 1) metal (Pd, Ni, and Cu) NWs and nanorods sheathed within MWCNT templates; 2) nanoparticles of palladium, rhodium, and ruthenium decorated onto functionalized MWCNTs. These highly dispersed nanoparticles are expected to exhibit promising catalytic properties for a variety of chemical or electrochemical reactions; 3) Cu or Cu-Pd nanocrystals deposited onto SiO2 or SiC NWs. Different types of nanostructures were achieved, including nanocrystal-NW, spherical aggregation-NW, shell-NW composites, and ”mesoporous” metals supported by the framework of NWs.