Colloidal Solutions Used as Template to Produce Nanocrystals
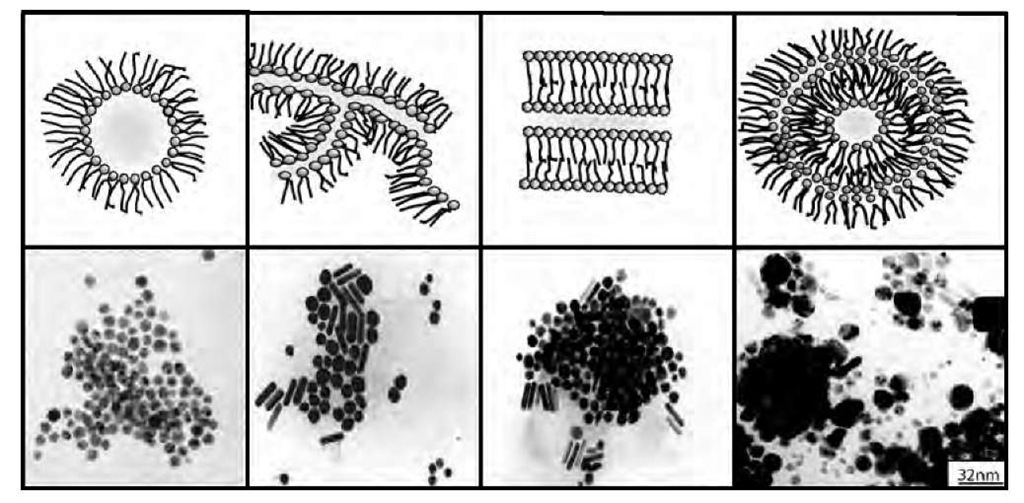
Instead of using reverse micelles, let us consider a phase diagram made of a functionalized surfactant such as Cu(AOT)2-water-isooctane. The confinement of the re-actant is still one of the major parameters. As (already) described above, the phase diagram markedly differs with the water content: At low water content, reverse micelles are formed. On increasing the water content, the system evolves to interconnected cylinders, then to an equilibrium between lamellae and interconnected cylinders, to an onion-phase region, and, finally, to reverse micelles.1-41,42-1 Hence, the polar volume fraction controls the shape of colloids. To make nanocrystals and to determine if the shape of the template controls that of the nanocrystals, water is replaced by hydrazine in the aqueous solution, keeping the same polar volume fraction colloidal shapes described above. Fig. 4 shows that the shape of the template partially controls that of copper nanocrystals.1-57-1 The crystallinity of these nanomaterials is very high. In the interconnected cylinder region, spheres and cylinders are formed: The cylinder structure is characterized by a fivefold symmetry.[58] In the region of the phase diagram consisting of an onion phase containing internal and external interconnected cylinders, a large variety of shapes are observed.[59- This control of the nanocrystal shape by that of the template has been recently confirmed by Simmons et al.[60- Syntheses of CdS nanoparticles in such colloidal assemblies characterized by various structures make it possible to vary the morphology of the nano-crystals from spheres to nanorods with a switch in the crystal structure from cubic to hexagonal. However, the role of templates is not as obvious as described above. Adsorption of ions, salts, and molecules has to be taken into account.
Fig. 4 Change in the shape of copper nanocrystals in various colloidal solutions differing by their structures. The colloidal solution is made of 0.1 M Cu(AOT)2 in isooctane and hydrazine is injected in the colloidal solution. At low water content (below 3), spherical reverse micelles are formed, inducing formation of spheres. On increasing the water content to w = 5, interconnected cylinders are produced with the formation of a mixture of spherical and cylindrical copper nanocrystals. In lamellar phase obtained at w =11, a mixture of spheres and cylinders is produced, whereas at w = 30, supra-aggregates are formed and a large variety of copper nanocrystals differing by their shapes are produced.
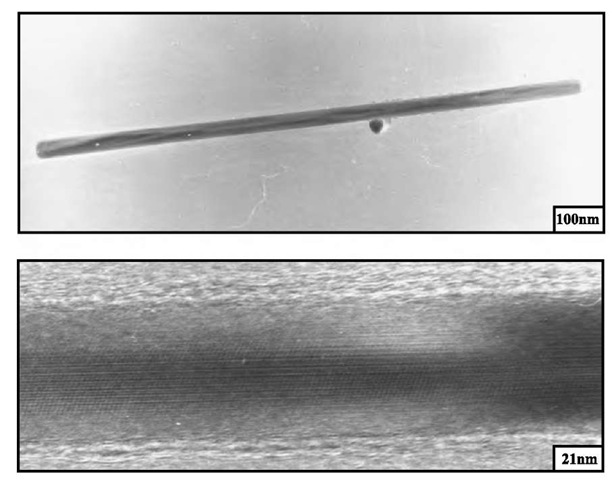
Fig. 5 TEM patterns at various enhancements of copper nanorods. Hydrazine is added to 0.1 M Cu(AOT)2 solubilized in isooctane in the presence of 10" 3 M NaCl and copper nanorods are produced at the end of the chemical reduction of Cu2+ by hydrazine.
Influence of adsorption of ions on nanocrystal growth
Let us consider the system described above [Cu(AOT)2-isooctane-water]. In the region of interconnected cylinders that are in equilibrium with a lamellar phase, small cylinders are obtained. Addition of a small amount of salt to the colloidal solution gives long copper nanorods with a high crystallinity in fivefold symmetry (Fig. 5)[61] and an aspect ratio controlled by the concentration of chloride ions in the microphase.[62,63- It has been demonstrated that this effect is mainly caused by the chloride ions. This is attributed to selective adsorption of these ions on (001) faces and to the fact that the growth is faster on the (111) faces. Formation of these nanorods is not observed, except with bromide, on replacing chloride by other anions[64-(Fig. 6). Note that with bromide anions, a rather large amount of cubic nanocrystals is formed, whereas with most other anions, mainly spherical objects are produced. From this, it is obvious that adsorption of a given anion enables control of the copper nanocrystal shape. These data have to be related to those obtained by Esumi et al.,[65] who produced gold nanorods by UV-visible irradiation of a gold salt solubilized in the bulk phase of normal micelles made of CTAC. The authors claim that direct micelles play the role of the template. It is difficult to understand this role: Gold ions do not interact with the micellar solution and are reduced photochemically. The chloride ions coming from the counterion of the surfactant could play this role, as observed above for copper nanorods produced in the presence of Cl", and allow the gold crystal growth along the 111 direction. These data can also be related to those published by Jana et al.,[66,67] who produced silver and gold nanorods with an aspect ratio controlled by the ratio of seeds and base concentrations in the presence of the CTAC surfactant. As mentioned, cubic KMnF3 nanocrystals are formed[53] from reverse micelles made from the CTAB surfactant and Cu(AOT)2-H2O-isooctane solution[63- in the presence of Br". Similarly, self-assembled monolayers (SAMs) used as templates and immersed in a solution containing bromide ions produce cubic cadmium sulfide nano-particles.[68- The environments of these various systems totally differ. The only common factor is the presence of bromide ions during the nanocrystal growth. However, it cannot be claimed that the general principle for growing cubes is that bromide ions have to be present during the process. As mentioned, bubbling hydrogen through an aqueous solution containing PtCl4" produces cubic platinum nanocrystals.1-52-1 From this, it can be concluded that cubic nanocrystal growth is more related to the presence of ions that absorb selectively than the template.
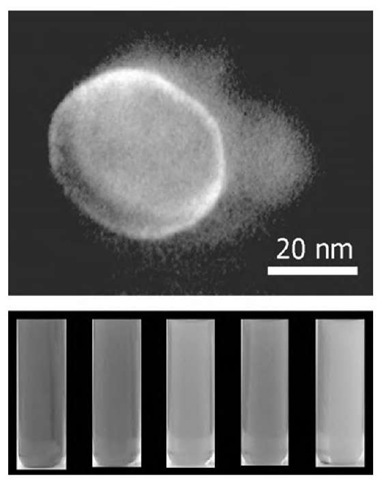
Fig. 7 Silver nanodisks characterized by various absorption spectra caused by change in the nanodisk size while keeping the same aspect ratio. A large volume of hydrazine added to 0.1 M Ag(AOT) is hexane. The relative amount of hydrazine controls the size of nanocrystals.
From the literature, a general agreement seems to emerge that control of the nanoparticle shape, via inorganic syntheses, needs to involve a mixture of two sur-factants.[73,74- This has been well demonstrated with various materials such as CdSe, cobalt, and Fe nanorods. The relative ratio of surfactants controls their aspect ratio. However, such claims are not always valid. In fact, the presence of a single surfactant is enough to control the dimensions of silver, gold, and copper nanorods. A convincing experiment showing that the selective adsorption of molecules is one of the major parameters in controlling the particle shape is the production of ZnTe and CdTe nanowires1-75,76-1 by the solvothermal process. Metals are solubilized in hydrated hydrazine, which is not only an electron transfer medium but also a strong electron donor. These authors claim that in this medium, which is, from my knowledge, a homogeneous solution, hydrazine plays the role of a template.
NORMAL MICELLES USED TO PRODUCE FERRITE NANOCRYSTALS
Fine magnetic particles, dispersed in a suitable liquid carrier (such as water, kerosene, diester, etc.), form a magnetic fluid. Their magnetic properties[77,78- cannot be analyzed without the inclusion of the effects of size, shape, surface, polydispersity, and interactions between particles. For this reason, a method for synthesizing the particles with good control of these parameters is necessary. Recently, we developed a new procedure to make ferrite nanocrystals that allows changing of the nano-crystal size while keeping the same surface area.
Divalent dodecyl sulfate [X(DS)2- (X=Fe, Co, Zn) is solubilized in aqueous solution and forms mixed oil-in-water micelles. A base is added to the micellar solution, is stirred for 2 hr, and, after centrifugation, the precipitate is washed several times with a solution of 50% water and 50% ethanol to remove the surfactant. Thus the powder obtained consists of ferrite nanocrystals. Depending on the type of ions, X, associated with Fe(DS)2 stoichiometric solid solutions of ferrite, is obtained. Hence, various materials such as Fe3O4,[10,79,80- y-Fe2O3[10,79,80- Cox-FeyO4,[81- and CoxZnyFezO4[82,83- were produced and the obtained material depends on the relative concentration of the reactants. Note that Fe3O4 is the reduced form of g-Fe2O3. Immediately after synthesis, Fe3O4 is produced. After a few hours, the Fe2+ ions are oxidized to Fe3+ and g-Fe2O3 is formed. With this procedure, a large variety of ferrite nanocrystals can be prepared. To obtain an alkaline magnetic fluid, the nanocrystals are dispersed in aqueous solution, whereas for a neutral fluid, the nanocrystals are coated with citrate ions and dispersed in water.[81,84] The nanocrystal size distribution is around 20-30%. Changing the surfactant concentration controls the particle size by a factor of 2 or 4. Whatever the fabricated material is, the nanocrystals structure is an inverted spinel. This procedure drastically differs from syntheses in homogeneous solution.[85-93] The major differences are:
1) The reactant concentration is two orders of magnitude lower than that in homogeneous solution.
2) A spinel structure can be obtained in the absence of Fe(III) at the starting point of the reaction, whereas the ratio Fe(II)/Fe(III) must be higher than 0.4 in homogeneous solution. In the latter case, with a high Fe(II) salt concentration and without Fe(III) deriva-tives,[94-96] the formation of Fe3O4 micrometer particles is observed. Their morphology depends critically on parameters similar to those described above (reactant concentrations, pH, ionic strength, etc.). Furthermore, it has been impossible to produce particles in the nanosize range when Fe(II) salt is used for the synthesis.
3) Changing the micellar concentration controls the particle size. In homogeneous solution, it is controlled by changing the type of salt (chlorides, nitrates, perchlorates, etc.), Fe(II)/Fe(III) ratio, pH, and ionic strength of the media. Such drastic changes in experimental conditions induce a large modification in the particle interface (hydroxide formations, etc.) and magnetic properties[92,93- of the nanocrys-tals. Conversely, using colloidal solutions makes it possible to produce nanocrystals with magnetic properties that do not depend on their coating.[81]
CONCLUSION
In the last decade, colloidal solutions were assumed to be very efficient templates for controlling particle size and shape. A large number of groups used reverse micelles to control spherical nanoparticles.[1,2] This makes possible determining the various parameters involved in such processes and demonstrates that they can still be considered as efficient nanoreactors with some discrepancies.1-97-1 There are fewer reports concerning the control of the particle shape and it is still rather difficult to determine the key parameters. They depend on the adsorption of salts, molecules, and procedure. Crystal growth on the nano-scale seems to follow behavior similar to that of the bulk phase with a marked dependence on pH. The latter is particularly important when some impurities are present in the growth medium because it influences, for example, the formation either of zwitter-ions or complex ions, the efficiency of which is greater than that of the initial impurity. These elements lead to a decrease in the growth rates of certain crystal faces. Most of the changes are based on the existence of a more or less epitaxial adsorption layer on the crystal. This layer is composed of solvents, impurities, or salts. Their precise roles are as yet uncertain. The changes are because of the differences between the growth rates of the various crystallographic faces. From this, it can be concluded that the template is not the key parameter in the shape control.
From these comments, we can ask why templates made of surfactants are quite effective in controlling the formation of nanospheres whereas rather large exceptions are observed for anisotropic shapes. This is probably because of the fact that colloidal templates are highly dynamic. The energy needed to produce spherical nanocrystals is less than that for producing anisotropic nanocrystals. A general method for controlling nanocrystal shapes through soft chemistry has not yet been found, but this does not mean that such a method cannot be discovered. To reach a final conclusion, we need more data and we need to compare the fabrication of anisotropic nanocrystals with various types of materials. Probably other approaches, which have yet to be found, are required. This is suggested by the fact that the fabrications of elongated ferrite nanocrystals in biological media and in vitro are completely different. In biological media, the mechanism of crystal growth is not well known. However, to produce similar nanomaterials in vitro, a very high base concentration is needed and the corresponding pH would induce the destruction of the biological media. This means that other ways exist and have to be discovered.