INTRODUCTION
During this last decade, because of the emergence of a new generation of high-technology materials, the number of groups studying nanomaterials has increased exponentially.1-1,21 The electrical, optical, and magnetic properties of inorganic nanomaterials vary widely with their sizes and shapes. Nanomaterials are used in several domains such as chemistry, electronics, high-density magnetic recording media, sensors, and biotechnology. This is, in part, because of their novel material properties, which differ from both the isolated atoms and the bulk phase. An ultimate challenge in materials research is the creation of perfect nanometer-scale crystallites identically replicated in unlimited quantities in a state than can be readily handled and can behave as pure macromolecular substances. Thus the ability to systematically manipulate these is an important goal in modern materials chemistry. Optimizing this ability requires an understanding of nanocrystal growth, which turns out to be a complex process. The essential first step in the study of their physical properties and the use of nanomaterials in various technologies is their production. Several approaches to manipulate inorganic nanocrystals have been undertaken. The major contribution was to produce spherical nano-crystals with a very low size distribution. Deposition processes include use of microwave plasma,[3] low-energy cluster beam deposition,1-4-1 inorganic chemistry,[5] ball milling,1-6-1 sonochemical reactions,1-7-1 sol-gel,[8] and flame by vapor phase reaction and condensation.1-9-1 In 1986, we developed a method based on reverse micelles (water-in-oil droplets) to prepare nanocrystals.[1,2] Normal micelles make it possible to produce ferrite magnetic fluids.[10]
To control the shapes of nanocrystals, several procedures are now being studied. Hard templates are employed to direct 1-D nanostructure growth. The nanometer-sized pores in membranes and zeolites are utilized to confine the growth of wires.[11-14] Alternatively, lithography and deposition are combined to create quantum wires on single-crystal surfaces.[15-17] Electrochemical synthesis is used to produce well-defined nanorods.[18-20] In 1993 and again in 1995, we were able to partially control the shape of nanocrystals by using colloidal solutions as tem-plates.[21,22]
In the following, we will concentrate mainly on nanocrystal growth in colloidal self-assemblies and describe discrepancies in the control of size and shape.
COLLOIDAL SELF-ASSEMBLIES OF SURFACTANTS
Direct Micelles
Surfactants are molecules with a polar hydrophilic head (attracted to water) and a hydrophobic hydrocarbon chain (attracted to oil). If a surfactant is solubilized in water, the chains tend to self-associate to form various aggre-gates.[23,24] Of course, if the solvent is able to solubilize simultaneously the polar head and the alkyl chains, no aggregates are formed. The shape of the surfactant plays an important role in forming the assembly. If the surfactant molecules have a very large polar head and a small chain, the chains tend to self-associate and form a spherical aggregate that is called a direct micelle. When the direct (or normal) micelle is formed at low concentrations, it is spherical and the length of the hydrocarbon chain and the size of the polar head fix its diameter. On increasing the surfactant concentration, various aggregate shapes are formed. The most commonly used surfactants are sodium dodecyl sulfate [Na(DS)], cethyl trimethyl ammonium chloride (CTAC), or cethyl trimethyl ammonium bromide (CTAB).
Reverse Micelles
If the surfactant has the shape of a champagne cork (small polar head and branched hydrocarbon chains), spherical water-in-oil droplets are formed. These are usually called reverse micelles.[25- They are a thermodynamically stable mixture of water, oil, and surfactant, where water and oil regions are separated by a surfactant monolayer. Because of the amphiphilic nature of the surfactant, numerous disordered or partially ordered phases are formed, depending on temperature and concentration.[26- The surfactant most frequently used is sodium di(2-ethylhexyl) sulfosuccinate, usually called Na(AOT). The water/iso-octane/Na(AOT) ternary-phase diagram shows a large zone where the reverse micellar phase is found. In this liquid-like phase, the ratio of water to surfactant concentration w = [H2O]/[Na(AOT)] determines the reverse micelle size. At w values lower than 15, water mobility is greatly reduced (bound water). Above w = 15, the linear increase of the water pool radius rw with w (from 4 to 18 nm) is explained by a geometrical model,[27] which assumes a constant area per surfactant molecule and that all surfactant molecules participate in the reverse micelle interface. They are able to exchange their water content during collision between two droplets. The volume of water added to the solution is the only parameter controlling the droplet diameter. Hence, the droplet size remains unchanged in various bulk oil solvents. The intermicellar potential, modeled by an adhesive sphere potential, depends on the particle diameter (d), the attractive range (A), and the sticky parameter (t-describing the attractive strength between two droplets.[28] The latter increases with the length of the bulk oil alkyl chain. It is related to the decrease in percolation threshold with oil chain length, and is explained in terms of an increase in intermicellar droplet interactions. This is caused by penetration of solvent molecules into the interface screening the AOT-alkyl chain interactions. In the case of long-chain oil solvents, steric hindrance does not allow solvent molecules to penetrate the interface, inducing an increase in attractive interactions.1-29-32-1 The kinetic exchange process[33] is directly related to the sticky parameter and to the modulus binding of the film at the water-oil interface. Hence, the solvent used tunes the kinetic exchange process: for short-chain solvents, the surfactant alkyl chain is well solvated and the micellar interactions are weak, inducing a low kinetic exchange rate constant. Conversely, large molecules are poor solvents for alkyl chains inducing strong interactions between micelles (i.e., high kinetic rate constants). Hence, by replacing cyclo-hexane by isooctane as the bulk oil solvent, the kinetic rate constant, at fixed droplet size, increases by a factor of 10.[34] These two properties (size and exchange process) make it possible by mixing two micellar solutions containing the reactants to produce nanomaterials.
Colloidal Self-Assemblies Made of Divalent Surfactant
Following our paper[35] in 1991, a great deal of work has been performed with divalent bis(2-ethylhexyl) sulfo-succinate [X(AOT)2]. We demonstrated that, at low water content, spherical reverse micelles are formed. It was found that, with Cu(AOT)2, Co(AOT)2, and Cd(AOT)2, on increasing the water content, spherical water-in-oil droplets turn into cylinders. This study has been extended by other groups; Eastoe et al.[36-39] confirm these data and show this for other surfactants such as Zn(AOT)2,Ni(AOT)2, and (C7H14)4N(AOT)2. In the oil-rich region, the phase behavior of copper(II) bis(2-ethylhexyl)sul-fosuccinate [Cu(AOT)2--isooctane-water is known over a wide domain.[40-43- When the surfactant is not totally solvated, the various structures are governed by the hy-dration of the head polar group with a progressive increase in the surfactant parameter s = vs/asls, where vs, as, and ls are the volume of the surfactant, the surface area, and the length of the alkyl chain, respectively. All these divalent surfactants behave similarly: At low water content, reverse micelles are formed. On increasing the water content, the system evolves to interconnected cylinders, to an equilibrium between lamellae and interconnected cylinders, to an onion-phase region, and, finally, to reverse micelles.[40-43- Hence, these structures are made with the same surfactant and differ by their shapes. In the region where the head polar group is totally hydrated, spontaneously formed thermodynamically stable emulsions in equilibrium with other microstructure phases are observed. These spontaneous emulsions are comprised of supra-aggregates,[43]—lamellar spherulites in which the interior and exterior are phases of interconnected cylindrical nanostructures. In another part of the phase diagram, clumps of interdigitated micelles are surrounded by an interconnected cylinder phase. The phase boundaries emerge qualitatively from elementary considerations that require only notions of local and global packing constraints.
SYNTHESES OF NANOCRYSTALS IN SELF-ASSEMBLIES DIFFERING BY THEIR SIZES AND SHAPES
In the oil-rich region, the reactants are confined in the water pool. Because of this, the chemical reaction (i.e., production of nanocrystals) takes place in the supersatu-ration regime, thus allowing the formation of particles having a very high crystallinity. Such self-assemblies are either droplets (reverse micelles), or bicontinuous phases such as interconnected cylinders or lamellar phases. In aqueous solutions, a functionalized surfactant is used to make normal micelles that are the chemical media for nanocrystal growth. In this case, the reactants are localized at the water-oil interface of the micelles, creating a supersaturation regime.
Syntheses in Reverse Micelles with Formation of Spherical Nanocrystals
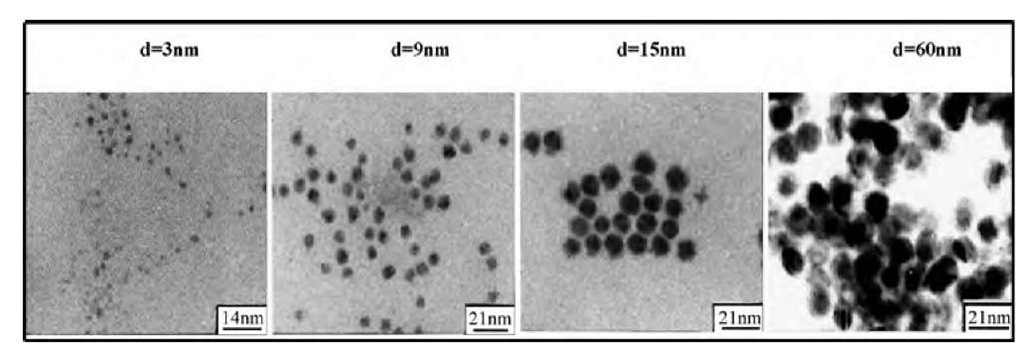
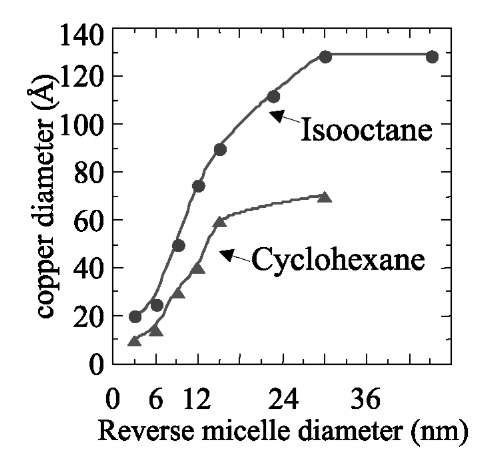
Fifteen years ago,[44- we discovered that reverse micelles are good candidates for templates. Coprecipitation reactions and chemical reduction occur in reverse micelles by using the two properties described above (change in the droplet size with the water content and micellar exchange process). Let us consider A and B solubilized in two mi-cellar solutions. On mixing them and because of the exchange process, A and B are in contact and react. Thus it is possible to fabricate a very wide range of spherical nanomaterials[1] such as semiconductors, metals, oxides, and various metal and semiconductor alloys. The control of the template size, by changing the water content, enables control of the spherical nanocrystal size.[21,44] Fig. 1 shows the control of copper nanocrystal size with the water content. It is of interest to note that this nano-reactor makes it possible to produce metal nanocrystals without any detectable oxide. The nanocrystals are characterized by a very high crystallinity when one of the reactants used is a functionalized surfactant (the surfactant has one of the reactants as the counterion). Otherwise, when the two highly hydrated reactants are solubilized in the two droplets, amorphous nanomaterials are formed and metals are produced in their oxide form. The size of the produced material, under the same experimental conditions, is not that of the droplet used as a template.[1,2] In fact, for II-VI semiconductors[45] (CdS, CdTe, and CdMnS), the particle size varies from 2 to 4 nm, whereas for metals, it increases from 2 to 6 nm for silver[46] and from 2 to 10 nm for copper[21] and silver sulfide.[47] This control of the particle size is obtained for the smallest water-in-oil droplets (varying from 0.6 to 6 nm), whereas for larger template sizes (from 6 to 12 nm), no changes in the particle size are observed (Fig. 2). This is well demonstrated for large numbers of nanocrystals and is explained in terms of water structure.1-48-1 An exception is found for silver sulfide nanocrystals with a linear increase in the particle size with that of the template and similar sizes of the droplets and the material.1-47-1 By changing the length of the solvent alkyl chain, we know that the droplet size remains the same, whereas the intermicellar interactions change. Syntheses at constant droplet size and in various bulk oil solvents induce a change in the produced nanocrystal size. This is observed for a large variety of nanocrystals and is well demonstrated with copper nano-crystals made in reverse micelles having isooctane and cyclohexane as the bulk phase (Fig. 2). This change in the particle size, keeping the same droplet radius, is explained in terms of efficiency in the exchange control process: Because cyclohexane is a good solvent for surfactant chains, intermicellar interactions between droplets and then the kinetic exchange rate constant are smaller with cyclohexane than isooctane.
Fig. 1 Change of the copper nanocrystal sizes with the reverse micelle diameter. Reverse micelles are made with 0.1 M Na(AOT) surfactant solubilized in isooctane. One solution contains 10" 2 M Cu(AOT)2, whereas the second one contains 2 x 10" 2 M hydrazine. The water content is fixed by the amount of water added to the solution and controls the droplet size. By mixing the two reverse micelles, copper nanocrystals are formed. A drop of solution is deposited on grid and the transmission electron microscopy (TEM) patterns are presented for various water droplet sizes.
Fig. 2 Variation of the copper nanocrystal size with the droplet diameters by using either isooctane or cyclohexane as the bulk oil solvent. The same procedure as described in Fig. 1 is used. Isooctane is replaced by cyclohexane. From the TEM pattern, the average diameter of nanocrystal sizes is measured.
Discrepancies in the Use of Reverse Micelles as Templates
The method described above makes it possible to obtain a very large number of spherical nanomaterials, indicating that reverse micelles are efficient templates. However, note that some of them do not exist in the bulk phase and others can never be produced. Hence, in equilibrium states, the solid solubility between Fe and Cu is negligibly small. Their mixing enthalpy is positive and they form no intermetallic compounds even though their atomic radii are quite similar. On the nanoscale, reverse micelles produce Fe/Cu alloys.[49- With semimagnetic semiconductors1-45-1 such as CdyMnj _y, it is possible to include, as in the bulk phase, 50% of Mn2+ ions in the CdS matrix, whereas with CdTe, there are no detectable manganese ions in the nanocrystals[50- and telluride nanorods are formed. No obvious explanations can be given. However, this indicates that physical chemistry in colloidal and homogeneous solutions differs.
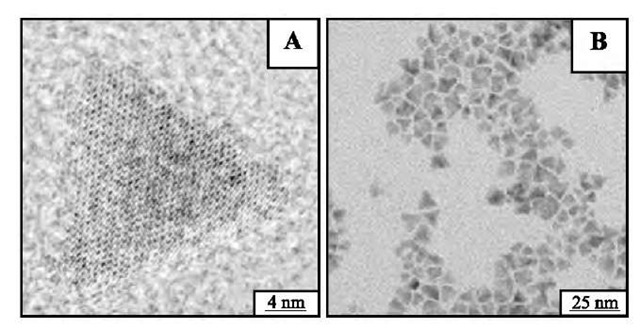
The experimental mode used to prepare the nanocrys-tals is one of the key parameters. This is also described below for controlling nanocrystal shape. Let us consider spherical reverse micelles made of functionalized surfactants such as cadmium bis(2-ethylhexyl) sulfosuccinate [Cd(AOT)2-. By adding a given amount of water, the water pool diameter reaches 10 nm. By replacing pure water with a solution containing sodium sulfide, spherical CdS nanocrystals are produced. Conversely, bubbling H2S diluted with nitrogen gas results in formation of flat triangles of CdS nanocrystals[51- (Fig. 3). High-resolution electron microscopy shows formation of well-defined monocrystals. The formation of flat triangles could be explained by the fact that the nucleation process is slower in using diluted H2S gas. This enables selective adsorption of hydronium (H+) on specific faces. Such nanocrystal growth cannot be explained by surfactant impurity:
Fig. 3 Triangles of CdS nanocrystals at various enhancements. Reverse spherical micelles of 0.1 M Cd(AOT)2 solubilized in isooctane at water content equal to 31 are submitted to a slow bubbling of H2S. CdS nanocrystals are formed.
The same reverse micelles produce spheres and triangles. This shape control by the presence of H+ in the solution can be related to the formation of cubic platinum nano-crystals1-52-1 from aqueous solutions containing PtCl2 _ and bubbled with hydrogen (H2). With time, hydrogen and chloride ions are formed in the solution and a precipitate of cubic platinum nanoparticles appears. It must be noted that the particles are well defined and faceted. Coalescence is prevented by selective adsorption of H+ or Cl_ on the facets. Other examples in the literature show formation of nonspherical nanoparticles: hence, spherical reverse micelles made of CTAB/butanol/octane produce cubic KMnF3 nanocrystals.[53] Details of the synthesis are not given and it is rather difficult to explain which parameter plays the determinant role. However, we have to keep in mind that bromide derivatives of CTAB are present during the synthesis. This will be discussed below. Similarly, elongated and rod-shaped BaCrO4 and CaCO3 nanocrystals are produced by using reverse micelles.1-54-57-1 This could be caused by selective adsorption on various faces during the nanocrystal growth of either reactive products or impurities coming from the preparation of functionalized surfactants used to form reverse micelles. From this, it is reasonable to conclude that reverse micelles can be used as nanoreactors to produce nano-particles. In most cases, a spherical template produces nanospheres. Hydration of the water pool, procedure mode, and size of the template control the nanocrystal growth. However, production of various species during the chemical reaction and the presence of some impurities prevent the control of spherical particles and induce the formation of nanoparticles having various shapes.