Nanocomputing
Buoyed by the success of preliminary experiments, it has been supposed that nanoscopic elements could act as ingredients in future computers.[33] Ordered arrays of nanocrystals, in principle, could be thought of as arrays of single-electron transistors (SETs), where the electrostatic interaction between neighboring SETs acts as a means of wireless communication. It has been suggested by Korotkov and Lent that simple logical operations can be performed on a circuitry consisting of arrays of SETs in the form of chains or cells with suitable insulating spacers.[34,35] An electric field applied in one direction polarizes the strings into either the 0 or the 1 state. Lent’s scheme, named quantum cellular automata, instead uses a square cell consisting of five nanocrystals to denote the state of polarization. Preliminary experiments to evaluate the schemes are currently being pursued. The realization that self-assembly driven fabrication process is not capable of producing defect-free structures has fueled a search for algorithms that can compute even with defective circuitry. Heath and coworkers have developed Tera-mac, a computer that works despite a high concentration of defects in its bank of microprocessors.^6 A more radical solution called amorphous computing aims to ”engineer pre-specified, coherent behavior from cooperation of large numbers of unreliable parts interconnected in unknown, irregular and time varying ways.”[37-39]
Catalysis
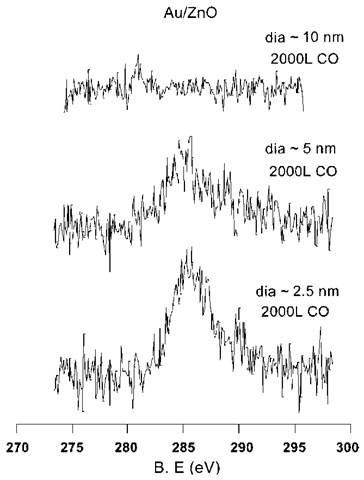
Nanocrystals consist of an unusually large fraction of surface atoms. For example, a nanocrystal of size 5 nm has about 80% of its atoms on the surface. This increases the surface area per unit mass and translates to a higher number of active site per unit mass and thereby makes nanocrystals extraordinarily important for catalytic applications such as in autocatalytic convertors. In addition to an increased surface area, nanocrystalline metals also posses high reactivity.[40-42] When combined with a suitable support, typically a semiconducting oxide surface such as TiO2 or ZnO, the nanocrystals exhibit an affinity for reactions that have no corresponding bulk analogs. For example, Goodman and coworkers have found that small Au nanocrystals supported on titania catalyze CO oxidation, with the catalytic ability being markedly size dependent.[43] Another study has found that Au nanocrystals supported on ZnO exhibit a tendency to adsorb CO.[44] The activity is size dependent and is exhibited only by small nanocrystals (diameters <5 nm, see Fig. 7). The increased activity of the small particles is attributable to the charge transfer between the oxide support and the particle. It is supposed that greater understanding of nanoscale charge transfer phenomena would contribute toward understanding of such catalytic processes.
OPTICAL PROPERTIES
The fluorescent properties of semiconductor nano-crystals have drawn wide attention because of their potential use as labels in fluorescence bioassays.[45-52] When compared to dyes currently in use, the emission from fluorescent nanocrystals is brighter and sharper. For example, Nie and coworkers have estimated that CdSe nanocrystals are 20 times brighter and 100 times more stable than a single rhodamine 6G molecule.[51] Further, the emission can be brought about by excitation in a broad range of wavelengths. It is therefore possible to excite nanocrystals of several different sizes simultaneously with a single source and obtain a well-resolved emission at different colors. In order that the nanocrystals are biocompatible, the nanocrystals need to be water soluble and possess pendant groups at the surface, that bind to biomolecules like proteins. These changes can be brought about by tailoring the ligand shell with small DNA fragments or mercapto acids. Several invivo and invitro fluorescence biochemical assays have been carried out with nanocrystalline markers.[45-47,51] A few studies have sought to exploit dependence of the plasmon absorption band on the dielectric constant of the surrounding medium in metal nanocrystals to detect binding events taking place at the ligand shell.[53-55] Thus, Au nanocrystals could colorimetrically determine the successful hybridization of oligonucleotide strands bound to its surface.[56,57] It has been proposed that colorimetric sensing of heavy metal ions could be obtained by the use of carboxylic acid terminated bifunctional thiols bound to metal nanocrystals.[58,59] The changes in the electronic absorption spectra of nm Ag nanocrystals capped with lipoic acid, following the addition of the heavy ions, Cu2+ and Fe2+, is shown in Fig. 8. Such a dampening also brings about a change in color. It is apparent that Cu2+ ions dampen the plasmon band more effectively than Fe2+. It has been suggested that very sensitive detection of nanoparticles could be carried out by measuring the light scattered by the parti-cles.[61-62] Yguerabid and coworkers have pioneered the use of large particles ~60 nm for carrying out biochemical assays. In contrast to detection in the liquid state, much progress has been made in surface plasmon based detection of binding events that occur over a bed of nanocrystals. Since 1990, Biacore has been marketing a surface plasmon resonance (SPR) based kit for carrying out immunoassays.[63]
Fig. 7 C (1s) core-level spectra of CO adsorbed on Au particles supported on a ZnO substrate. The feature at 285 eV corresponds to molecularly absorbed CO. The diameters have been obtained from the metal coverages.
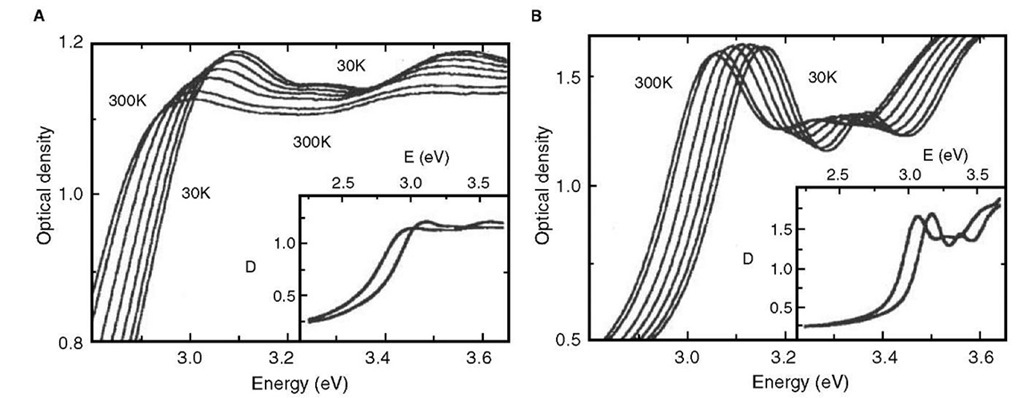
Another dimension has been added to the optical properties of nanocrystals by the recognition that the optical properties of a superlattice of nanocrystals could be different from that of the individual nano-crystals owing to interparticle interactions.[64] Typical spectra showing such a change in the case of CdSe nanocrystals are shown in Fig. 9. When present in close-packed organization, the absorption spectra of the nanocrystals are broadened and red shifted. This change has been attributed to interparticle dipolar interactions.[65] Bawendi and coworkers have studied such changes in the ensemble of CdSe nanocrystals of different diameters and have obtained evidence for long-range resonance transfer of electronic excitation from smaller to bigger nanocrystals owing to dipolar interactions.[66] In a noteworthy experiment, Weller and coworkers, prepared drop cast films of giant CdS clusters of the form Cd17S4(SCH2CH2OH)26 and Cd32S14(SCH2CH(CH3)OH)36 with diameters of 1.4 and 1.8 nm, respectively. Further, an integrating sphere was used to collect absorption data, thereby virtually eliminating errors from inhomogeneities and size distributions.[67] The experiments due to Weller also support the idea of dipolar interaction leading to the red shift and broadening. Signatures of such interactions have also been seen in the case of CdS multilayer deposits.[68] The interparticle interactions, however, could range from weak dipolar interactions to strong exchange interactions based on the interparticle separation. Delocalization of the electronic states of nanocrystals in ensembles due to exchange interactions have been observed in experiments with CdSe nano-crystals. Gaponenko and coworkers have shown that the optical properties of an ensemble of small (~1.6 nm) CdSe nanocrystals are similar to that of bulk CdSe because of complete delocalization of the electronic states of individual nanocrystals.[69] Dipolar coupling interactions in metal nanocrystal assemblies such as in linear rows lead to strong optical anisotropy. The transmitted light depends on whether it is polarized parallel or perpendicular to the rows of nanocrystals. It has been suggested that such material could be useful as polarizing filters. Dirix et al. have pioneered a simple method of preparing such assemblies. Their method involved stretching a polyethylene film impregnated with Ag nanocrystals. Dirix et al.[70] Atwater and coworkers have suggested that coupled plasmon modes could lead to coherent propagation of electromagnetic energy, i.e., a row of metal nanocrystals could act as a nanoscale waveguide. Using an array generated by means of e-beam lithography, they have provided a proof of concept demonstration.
Fig. 8 Electronic absorption spectra of nm Ag nanoparticles showing changes accompanying the addition of (A) Cu2+ and (B) Fe2+ ions. The concentrations of the ions are indicated.
Fig. 9 Absorption spectra of thin films of close-packed (A) and isolated (B) CdSe nanocrystals at different temperatures (from right to left curves): 30, 80, 130, 180, 230, 280, and 300 K. The insets show the full-range spectrum of optical density D for the lowest and highest temperatures. A red shift and broadening of the peaks is seen in the case of close-packed films.
!Several polymer/polyelectrolyte-nanocrystal hybrid devices have been fabricated seeking to exploit the electro- and photoluminescent properties of nanocrystalline media.[72-81] Device fabrication in all these cases is by low-cost self-assembly based techniques. These devices utilize thin films of these hybrids obtained either by multilayer deposition or drop/spin casting methods. Thus, "solar cells” have been made from poly(2-hexylthio-phene)-CdSe nanorod multilayers, lasers from drop cast films of CdSe-titania composites, and infrared emitter from multilayers of HgTe and poly(diallyl dimethyl ammonium chloride) (PDDA). White light electroluminescence is seen in multilayers of CdSe-CdS-poly(phe-nylenevinylene), CdTe-PDDA. White light emission has also been obtained from drop cast films consisting of a mixture CdSe-ZnS, CdS-ZnS, and polylaurly-methacrylate. The characteristic of all the above devices can be changed by altering the nanocrystal size.
MAGNETIC PROPERTIES
It is well known that in the nanometric domain, the coercivity of magnetic nanocrystals tends to zero.[82,83] Thus, the nanocrystals behave as superparamagnets with no associated coercivity or retentivity. The blocking temperature that marks the onset of this superpar-amagnetism increases with the nanocrystal size.[84-86] This scenario, however, changes in the case of interacting nanocrystals where the interparticle interaction and, hence, its magnetic properties can be tuned by varying the interparticle distance. Thus, lattices of interacting magnetic nanocrystals are considered important in the future magnetic storage devices. Further, the magnetic moment per atom is seen to increase as the size of a particle decreases.[87]
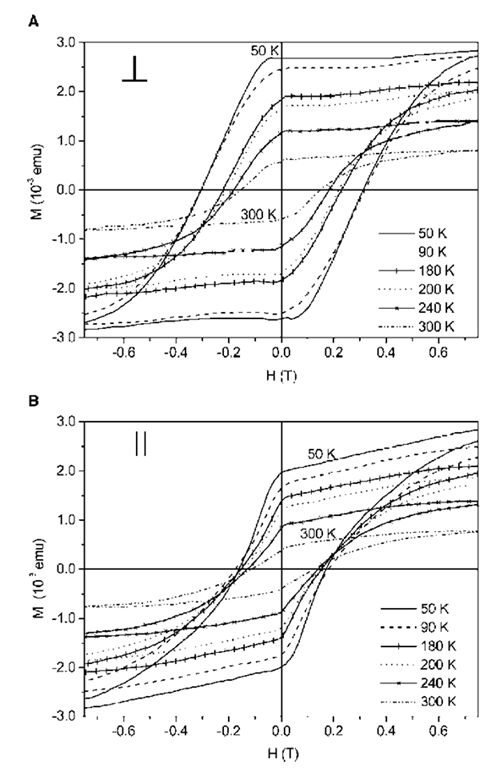
Nanocrystals of Co when organized into two-dimensional arrays exhibit a higher superparamagnetic blocking temperature compared to isolated nanocrys-tals, i.e, they display a higher resistance to thermal reversal of their spins than when they are isolated.[88] Sun et al. report a lattice of nanocrystals each consisting of a Fe core and a Pt shell prepared by heating Fe-Pt alloy nanocrystals.[89] Following phase segregation, the interaction between the nanocrystals increased leading to a ferromagnetic film capable of supporting high-density magnetization reversal transitions (see Fig. 10). Exchange spring magnets — nanocomposites that consist of magnetically hard and soft phases interacting via magnetic exchange coupling, have been made by carefully annealing the mixed nanocrystal array consisting of Fe-Pt and Fe3O4[90] The easy magnetic axis of nanocrystals can be aligned by applying a magnetic field during evaporation of the colloid on a substrate to obtain films with high magnetic anisotropy.[91-93] Thus, ferromagnetic films with parallel anisotropy have been made of superpara-magnetic g-Fe2O3 nanocrystals.[91] By tuning the sub-strate-nanocrystal interaction, the films can be made to exhibit perpendicular anisotropy (see Fig. 11).[92] Properties such as coercivity, anisotropy can be tuned by altering the size of the nanocrystals, the film thickness or by suitably doping the nanocrystals with magnetic ions.[91-93]
Fig. 10 Magneto-resistive (MR) read-back signals from written bit transitions in an array of 4 nm-diameter Fe48Pt52 nanocrystals. The line scans reveal magnetization reversal transitions at linear densities of (A) 500; (B) 1040; (C) 2140; and (D) 5000 flux changes per millimeter.
Fig. 11 Hysteresis loops from a film of g-Fe2O3 deposited on Si(100) substrates at various temperatures with the substrate held (A) perpendicular and (B) parallel to the applied field direction. The increased coercivity along the perpendicular direction indicates perpendicular anisotropy caused by orientation of the easy magnetic axis of g-Fe2O3 nanocrystals perpendicular to the substrate.
Biomedical Application of Oxide Nanoparticles
Magnetic oxide nanoparticles are considered as biocompatible as they possess no known toxicity. Superparamagnetic oxide nanoparticles have therefore found several biomedical applications.[94] By tailoring the ligand shell of magnetic particles to attach to a specific target molecule in a solution containing several different entities and subjecting the liquid to a magnetic field, separation or enhancement in concentration could be achieved. Such aids are rather important in biochemical experiments where very low concentrations are generally employed. For example, by employing red blood cells labeled with iron oxide nanoparticles, the sensitivity of detection of malarial parasite was shown to be enhanced by Paul et al.[95] It has been proposed that hyperthermia—magnetic field induced heating of superparamagnetic particles, could be used to destroy diseased cells and thereby treat cancer. The particles are dispersed in the affected cells and an external AC magnetic field applied to bring about heating of the particles to cause destruction of the cells. Numerous studies have been carried out toward achieving this objective.[94,96,97] Magnetic nanoparticles also find application as magnetic resonance imaging contrast agents. This technology has been commercialized and is widely available.[94]
CONCLUSIONS
Properties of metals and semiconductors become size dependent at the nanoscale because of associated changes in the electronic structure and charge transport. Specifically, electronic, optical, and magnetic properties of nanocrystals have gained importance in view of their potential applications. Small metal nano-crystals with discrete charge states find applications in highly sensitive electrometers and memory devices. Similarly, semiconductor nanocrystals of varying optical gap find applications in photonic materials and fluorescent markers in biochemical assays. Bimetallic and oxide nanocrystals with superparamagnetic domains have demonstrated potential as recording media. The latter especially has gained importance in cancer treatment.