NANOCRYSTALLIZATION DURING MELT QUENCHING
As noted in Fig. 4, it is possible to encounter conditions that represent the maximum nucleation rate (i.e., the minimum time for the onset of nucleation or the nose of the C curve) during continuous cooling. One important requirement to achieve a maximum nucleation density is the removal of potent heterogeneous nucleation sites so that the alloy melt may be cooled to the temperature yielding the maximum nucleation rate without prior crystal formation. For homogeneous nucleation the temperature for a maximum nucleation rate is about 2/3 Tm.[12- The schematic illustration in Fig. 4 does not provide a perspective on the sensitivity of the micro-structural scale to cooling rate at temperatures near the maximum nucleation rate temperature, but this important feature of nanocrystallization can be developed by considering a melt-quenched nanostructure.
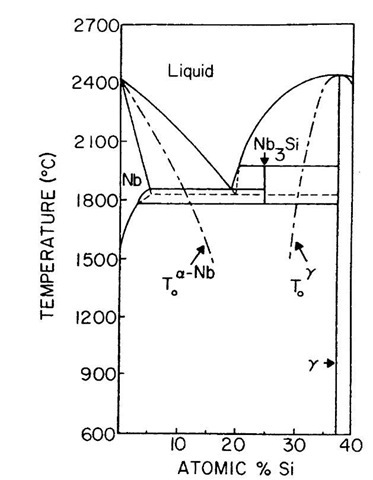
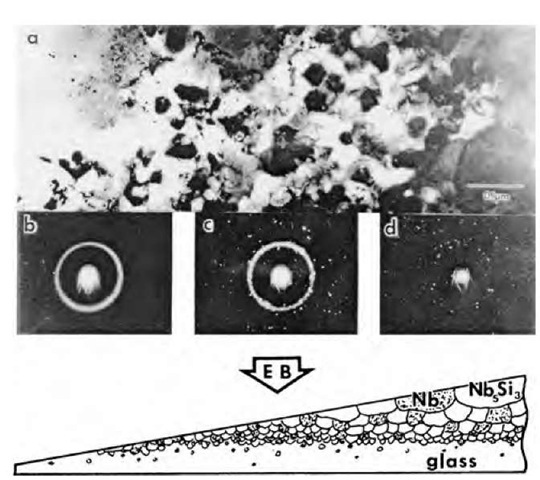
For Nb-rich alloys in the Nb-Si system the main solidification reaction is a eutectic yielding Nb+Nb3Si as indicated in the phase diagram in Fig. 6.[47- The mic-restructure that is developed following melt spinning is revealed in Fig. 7 for a wedge-shaped sample that was produced by thinning the ribbon of a Nb-25 at.% Si alloy from one side (opposite from the wheel side) the most rapidly cooled region adjacent to the wheel is amorphous. With increasing distance from the chill surface the onset of crystallization is characterized by nanoscale grains with a size of about 15 nm. The fine-grain structure is a mixture of Nb and Nb5Si3, but the size scale increases to about 100 nm over short distance within the 20-p.m-thick ribbon before the equiaxed structure evolves into a dendritic morphology.
A common occurrence in the synthesis of nanostructures during rapid solidification is the absence of a phase that according to the equilibrium phase diagram should be present. This occurs when the missing phase is subjected to kinetic limitations in nucleation and/or growth. In the absence of a stable phase, solidification is then governed by a metastable phase diagram. This diagram is constructed from the equilibrium diagram in Fig. 6 where the Nb3Si phase is suppressed and where the Nb5Si3 (designated g) liquidus extends below the L+Nb5Si3!Nb3Si peritectic isotherm and intersects the Nb liquidus to yield a metastable L! Nb+Nb5Si3 eutectic. For alloys near a peritectic reaction, solidification of the high-temperature phase (in this case, Nb5Si3) can continue below the peritectic temperature while the remaining liquid develops only slight undercooling with respect to the low-temperature phase (in this case, Nb3 Si). Hence, phases that are ordinarily formed by pe-ritectic reactions may often be absent in nanocrystalliza-tion reactions.
Fig. 6 Phase diagram for the Nb-Si system showing the metastable extension of the Nb5Si3 (i.e., g) liquidus to form a metastable eutectic L! a-Nb + Nb5Si3. This extension is relevant when the Nb3Si phase is absent. Also shown are approximate T0 curves for the transformation of liquid to a-Nb and to g.
A useful method for examining the thermodynamic options available to an alloy undergoing nanocrystalliza-tion is to examine the T0 curves for the various liquid-to-crystal transformations in a system. Schematic T0 curves are included for the Nb and Nb5Si3 (g) phases in Fig. 6. The T0 curves place a bound on temperatures and compositions where partitionless solidification is possible.[13]
Regardless of the level of undercooling that is achieved during rapid quenching, a range of compositions may exist between adjacent T0 curves where partitionless solidification is impossible and crystal growth must involve the more difficult process of diffusional solute redistribution into a mixture of solid phases.[48] Because of this difficulty, glass formation or nanocrystallization is most likely in this range, which was observed to be centered near Nb3Si as indicated in Fig. 6.
The synthesis of a two-phase nanostructure clearly involves copious nucleation. One factor that can increase the opportunity for high nucleation rates is the relatively slow growth for crystals that require solute redistribution. Furthermore, during continuous cooling, the rate of re-calescence after the initial nucleation event depends on the crystal growth rate. If it is slow, the melt may be undercooled further, permitting a rapid rise in the nu-cleation rate.[12] Moreover, the two-phase nanostructures that are developed during melt spinning identify an important class of metastable microstructure that is available in the form of very high grain densities of the equilibrium phases that were observed to range from 1018 to 1021 m~3. A high density of grains for a two-phase mixture is metastable because of a high incoherent in-terphase boundary area. For example, with a grain size of 0.12 mm and an interphase boundary energy in the range of 500-1000 mJ m- 2 that is typical for incoherent interfaces, the excess free energy due to the polycrystal-line grain structure is 120-150 J mol-1. However, for nanoscale grains with a size of 15 nm the free energy increment increases to the range of 800-1600 J mol- 1. Thus, with nanostructured grains the level of metastability is comparable with that associated with nonequilibrium crystal structures.1-14-1
Fig. 7 (a) Microstructure of a Nb-25 at.% Si melt-spun ribbon near the TEM foil edge (the left-hand side of the micrograph) for a specimen polished from one side; (b) SADPs positioned under the areas from which they were taken; (c) a schematic drawing showing the microstructure across the ribbon (EB, electron beam).
The devitrification of an amorphous phase is a fairly well recognized approach to the synthesis of a nanocrys-talline grain structure. The attainment of an equiaxed grain structure resulting from an isotropic growth is characteristic of a polymorphic type of crystallization reaction. In this sense the evolution of an equiaxed two-phase nanocrystalline grain structure represents a distinct pattern of noncooperative growth that does not appear to be readily achieved by continuous heating or isothermal annealing devitrification treatment. However, the production of a high nucleation density for each phase appears to require a high rate of heat extraction as well as diffusional growth limitations so that the two-phase nanocrystalline structure is limited in spatial extent because of a relatively narrow range of favorable processing conditions.
NANOCRYSTAL CATALYSIS
The limited experimental information available indicates that the nucleation process during primary crystallization of amorphous alloys is usually heterogeneous in nature, but the origin of the active catalytic sites has not been identified in all cases.[49- For example, it has been established that, with several amorphous Fe alloys, the development of a high density of Fe nanocrystals is strongly promoted by the addition of small amounts of certain solutes such as Cu.[50- In fact, high-resolution transmission electron microscopy (TEM) and atom probe field ion microscopy (APFIM) observations indicate that Cu solute allows for reactions that act to catalyze Fe nuc-leation.[51,52- In amorphous Al-base alloys it appears that a comparable nucleation catalysis behavior can be observed with both soluble and insoluble additions. For example, the addition of 1 at.% Cu to amorphous Al-Ni-Sm has been reported to yield an Al nanocrystal density approaching 1023 m-3 with diameters of 5-7 nm.[53- Similarly, the incorporation of insoluble nanosized Pb crystalline particles in an amorphous matrix is effective in catalyzing the crystallization of Al nanocrystals and yields a significant increase in the total number density of nanocrystals.1-54-1 The catalysis behavior highlights the opportunity for the controlled synthesis of bulk alloys with an ultrahigh number density of nanoscale dispersoids.
MECHANICALLY INDUCED CRYSTALLIZATION OF AMORPHOUS PHASES
One consequence of the metastability of nanocrystalline and amorphous phases is that the structure and properties of the materials can depend on the processing pathway. This pathway dependence offers the chance to obtain phases with novel characteristics that cannot be achieved by melt quenching. For example, nanocrystallization reactions during cold rolling occur after continued folding and rolling of initially crystalline or amorphous multilayer samples, as illustrated schematically in Fig. 8. The repeated folding and rolling process can yield a true strain in the multilayer sample of the order of 100.[55-The kinetics of this crystallization process appears to be linked to the initial size distribution and density of the quenched-in nuclei in the amorphous matrix. In some cases, for example in melt-spun Al92Sm8, the sample fully crystallizes during rolling.[56- Amorphous alloys that follow the nucleation-controlled solidification pathway and therefore have no significant cluster concentration are considerably more stable against a rolling-induced crystallization reaction.[57- The observed primary crystallization of marginal glass formers during initial rolling implies an atomic transport of the constituents through the amorphous matrix. Nanocrystals can also be induced by deformation in bulk glass-forming alloys without any thermal annealing.[58- The redistribution of solute atoms during crystallization appears to be characteristic of a driven system where an athermal mechanical process during shear deformation controls the trans-port.[59-61- A more complete understanding of deformation-catalyzed nanocrystallization should also consider the nonequilibrium nature of the rolling process as well as the structural metastability of the amorphous phase. Understanding the nature of shear-induced crystallization is thus not solely a matter of focusing on the processing conditions and their influence on the structural modifications, but necessitates a refined knowledge of the amorphous structure before deformation.
Fig. 8 A schematic illustration of the cold-roll and fold process. A multilayer of elemental foils with foil thicknesses between 7 and 25 mm is reduced by 50% with each rolling pass.
CONCLUSION
The development of nanostructured materials can be achieved in bulk form by the use of amorphous phases as effective precursors. Indeed, the controlled primary crystallization of amorphous alloys yields essentially a nano-phase composite of nanocrystalline primary phase dispersed within an amorphous matrix. It is remarkable that the nanocrystal number density can achieve high levels of 1021 to 1023 m~3 at primary phase volume fractions approaching about 30% to yield ultra high strength. An equally remarkable fact is the relative high thermal stability of the nanophase composite. This is truly a novel microstructure that has revealed challenging basic issues on the governing reactions kinetics that control the structure synthesis and performance. At the same time, alternative synthesis routes have been identified based on deformation-induced alloying and glass formation that can be adapted for processing of bulk glasses. The deformation response of amorphous alloys is sensitive to the processing during synthesis and to the influence of quenched-in crystallites.