INTRODUCTION
Active metal powders are extensively used as fuels in most solid rocket propellants because of the high energy produced during their combustion. The specific impulse (Isp) of the rocket engine is proportional to (Tc/M)1/2, where Tc is chamber temperature and M is molecular weight of combustion products. Thus the best propellants are those that produce the highest combustion temperature and the smallest possible molecular weight of the combustion products. Therefore the best oxidizers are fluorine and oxygen and the best fuels are lithium, beryllium, boron, aluminum, and magnesium. Lithium is extremely reactive and beryllium is extremely toxic so these are impractical in rocket applications. That leaves boron, aluminum, and magnesium powders as primary candidates.
Aluminum is a major ingredient in solid rocket fuels, often combined in a rubbery binder along with particles of oxidizer. When burning aluminum in solid propel-lants, the energy utilized can be diminished because the droplets agglomerate, producing larger droplets and slower combustion that can occur too late (after the nozzle) to be effective. The agglomerates, although partially oxidized, often slag up on the internal surfaces of the engine, reducing combustion efficiency and weighing down the vehicle.
As with solid propellants, adding aluminum to liquid fuels would also provide a theoretical advantage in higher volumetric energy density, but the metal must be uniformly dispersed and remain so in the hydrocarbon. As with solid propellants, aluminum combustion must be rapid enough so that it is consumed within the rocket engine. The most effective means of achieving complete combustion is to use powders with particle sizes at least an order of magnitude or two smaller than the metal powder ordinarily used in solid propellants. This article focuses on Alex® nanosize aluminum particles manufactured by the electroexplosion of metal wire (”EEW”) and its use in liquid and solid rocket propellants.
BACKGROUND
Metal Powders for Solid Rocket Engines
Boron has been considered for many years as a candidate solid rocket fuel as it has a high energy content on both a gravimetric (Isp) and volumetric (density Isp) basis. In practice it has been difficult to realize these advantages as the combustion is severely hindered by a layer of oxide layer (B2O3) on the particle surface and boron’s vapor pressure is too low to escape the droplet, limiting the gas-phase oxidation to a much slower heterogeneous surface reaction.[1-3]
Aluminum has always been recognized as a highly energetic reactant for solid rocket fuels and one that can be more practically applied than boron. Because its specific gravity is high (2.7 g/cm3) relative to organic fuels, it is particularly advantageous for increasing density specific impulse, thereby reducing the size and therefore the weight of the rocket. It is generally used with ammonium perchlorate (AP) as a solid oxidizer and the two solid phases are held together with a rubber base binder such as hydroxy-terminated polybutadiene (HTPB) resin. Aluminum and its oxides are nontoxic and nonpolluting. While AP has been the oxidizer of choice, it is a groundwater pollutant and it also produces large quantities of hydrogen chloride in rocket exhaust. Newer and more energetic oxidizers such as ammonium dinitramide (AND) are being considered, primarily because they do not produce halogens in the exhaust. Also, there are efforts to replace HTPB with more energetic binders such as glycidyl azide polymer (GAP).
Metal Powders for Liquid Rocket Engines
When burning aluminum in solid propellants, the delivered performance is affected because it agglomerates while molten. Duterque[4] collected the ash of aluminum from a solid propellant burn and found that it contained a significant amount of unburned aluminum. Despite this deficiency, aluminum is quite attractive and it is used widely in solid propellants, particularly as the aluminum oxide particle reduces or suppresses combustion instability by damping of combustion waves.[5]
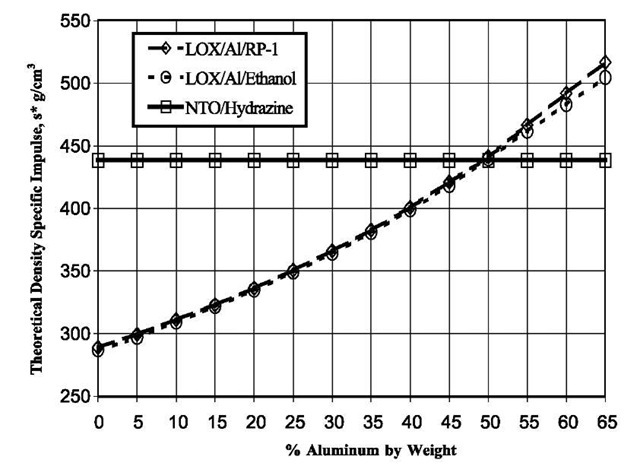
Fig. 1 Theoretical density specific impulse (vacuum, 100:1 expansion ratio) of aluminized bi-propellants.
Dispersing metal particles as a gel in liquid propellants have been studied analytically and experimentally for more than 69 years[6] because of the higher specific impulse afforded by adding active metals such as aluminum. NASA[7-13] studied its use into gelled RP-1 (kerosene) for bi-propellant liquid propellant systems. It has also been considered as an additive to liquid hy-drogen[14] because it would thicken the hydrogen, increasing safety in the event of leakage. Palaszewski and Rapp[11] suggest that given the suitable rheological tailoring, metallized propellants offer tremendous safety advantages. Design studies conducted for NASA missions show that aluminized gelled RP-1 with liquid oxygen can deliver rocket engine efficiencies that are comparable to that of traditional liquid propellants. Palaszewski and Rapp[11] discuss how using O2/RP-1/Al gel propellants as a replacement for the liquid rocket booster in the space shuttle boosters results in shorter boosters for the same payload size due to the increased propellant density. This in turn has the potential to deliver a higher payload mass over its solid counterpart. Fig. 1 shows the volumetric energy density calculated using the Propellant Evaluation Program (PEP) code[15] for aluminized RP-1 and ethanol fluids and compares these two fuels with nitrogen tetroxide/hydrazine, a highly energetic bi-propellant but one that is toxic and environmentally unfriendly. The computed energy available increases consistently with increasing aluminum content.
Unfortunately, conventional aluminum powder, when burning in liquid hydrocarbon fuel, also agglomerates as in the case of solid propellants, reducing delivered specific impulse (Isp). Wong and Turns[16] noted significant Al agglomeration in burning JP-10 resulting in inefficient combustion. And as in the case of solid propellant engines using aluminum, this agglomeration would result in unburned combustion products deposited on engine walls.
Metal Powders in Liquid Monopropellants
In the late 1950s and early 1960s, Atlantic Research studied the combustion of Arcogel, a gel of aluminum powder, ammonium perchlorate with dioctyl adipate as a liquid carrier. They found that such mixtures had adequate (6 months) shelf life and good stability under high acceleration loading and vibration. Arcogel was extensively characterized and found to be nondetonable and insensitive to ignition. They also conducted small motor tests. However, they found the viscosity to be unac-ceptably high at low temperatures (— 55°C). In the early 1990s, this work was continued,1-17-1 evaluating nitrated ester/AP/Al, hydrogen peroxide/Al, and HN/water/Al gels. All were found to have limited promise because of their sensitivity to premature ignition or detonation.
Environmental Issues
Regulatory environmental requirements are forcing new choices for rocket propellant ingredients. For instance, ammonium perchlorate (AP), the oxidizer of choice for solid propellants, is falling out of favor because hydrogen chloride is a significant combustion product. Toxicolog-ical constraints are also affecting the choice of liquid propellants. For instance, hydrazine, which is extensively used, is very toxic, as is nitrogen tetroxide. Threshold limit value (TLV) concentrations for hydrazine are fractions of a part per million, often below the limits of detection (LOD) of continuous monitoring instruments. Those working with this fluid often have to wear completely encapsulated suits. In addition to AP, there are two other inorganic oxidizers that have potential for solid propellants—ammonium nitrate (AN) and ammonium dinitramide. Another oxidizer being developed in the Netherlands is hydrazinium nitroformate (HNF).
Fluids such as ethanol[18,19] and methanol[20] are being considered as nontoxic options in bi-propellant system. Boeing[21'22] reported on the hazardous and costly operations associated with monomethyl hydrazine (MMH) and nitrogen tetroxide propellants related to the Space Shuttle Orbiter OMS/RCS system which culminated in NASA’s selection of ethanol/LOX as the bi-propellant. They designed such a propellant system and projected improvements in vehicle weight, complexity, and operational cost. They believe that the design solutions are applicable to other space-based cryogenic propulsion and power generation systems. Aluminizing these nontoxic fuels could substantially enhance propellant energy density without causing any environmental or toxic concerns.
Effect utilization of aluminum’s combustion energy in propellants resolves a number of problems—improved performance, particularly on a volumetric basis, and achieving that with no penalty as a pollutant or a toxin.
NANOMETAL POWDER FUELS
Many of the difficulties related to inefficient burning of aluminum powder in solid (as well as liquid) propellants could be ameliorated if the particle size of the powder were small enough. Nanosize aluminum was first prepared in the early 1970s by Russian scientists in Siberia,[23] who formed the powder by electroexploding metal wires (EEW). The EEW method is described in a separate article of this topic. Most of the Russian work was focused on producing nanosize aluminum for its potential use as a metal additive for rocket propellant fuel.
Electroexploding metal wire aluminum powder (Alex®) consists of spherical particles that are fully dense and about 100 nm in size. Because they are so active, during collection they form hard agglomerates. Because these particles are potentially pyrophoric, the final step in the manufacturing process involves controlled oxidation with dry air to form a passivating layer approximately 3 nm thick. Oxygen analysis shows the coating to be about 92-95% aluminum (between about 85% and 89% as oxide). X-ray diffraction shows the crystallites to be essentially aluminum, while the coating has been found[24] to be principally aluminum oxide, with some aluminum nitride and some aluminum oxy-nitride. The passivation step is controllable and thinner passivation layers are readily obtained, but the U.S. Department of Transportation (DOT) and International Air Transport Association (IATA) prevent air shipment of pyrophoric materials, so sufficient coverage is necessary to assure no pyrophoricity.
When ignited in air Alex® appears to have two separate ignition steps. The first occurs at lower temperatures (400-500°C) and a second where the powder burns white hot. Mench et al.[25] studied the thermal behavior of Alex® powder and compared it to micron-size aluminum. Their data show that Alex® powder has very sharp exo-therms when heated in air, oxygen, or nitrogen. These occur well below the melting point (660°C) of aluminum, while 20-p.m-size aluminum does not react with oxygen or air until about 1000°C.
A coated version of nano-aluminum (L-Alex®) is based on the replacement of the oxide coating by reaction with palmitic acid to form a monomolecular layer of palmitate. This version was tested by boiling the coated Alex® in water for an hour and the resulting coating appears to protect the metal, while ordinary Alex® would have reacted within minutes. Accelerated aging testing showed[26] L-Alex® to be superior in moisture and oxidation resistance to conventional Alex®.
Alex® as an Additive to Solid Propellants
Ivanov and Tepper[23] reported a 10- to 20-fold increase in burning rate if air-passivated Alex® was substituted for ordinary propellant grade aluminum when mixed with ammonium perchlorate powder (no binder). Sigman and co-workers[27] also reported that the burning rate of Alex® with AP (again no binder) was 10 times greater than with other micron-size aluminum powders. Chiaverini et al.[28] reported that Alex® when added to HTPB binder produced a substantial increase in the burning rate in oxygen.
The agglomeration of aluminum droplets has been studied by Simonenko and Zarko[29] who noted that there was a growth in aluminum oxide particles collected from the combustion products of a solid propellant grain as compared to that of the original particle size of the aluminum. They also noted that there was a substantial amount of aluminum left within the residual products they collected. In a separate study Glotov and co-workers[30] noted that the replacement of commercial aluminum by Alex® in quantities as small as 8 wt.% increases burning rate and decreases agglomerate size as well as increases the amount of oxidation. For example, total replacement of commercial aluminum by Alex® increases combustion efficiency to 99.2% as compared to 94.28% for coarse aluminum.
There have been considerable studies and modeling of the combustion of aluminum in a solid rocket engine. The Brooks and Beckstead model[31] estimates that the life of the particle is proportional to the particle size raised to the power of n with nominal values of approximately 1.5 to 1.8.[32] The life of a 35-p.m particle is about 6 msec. Extrapolating the Brooks and Beckstead equation for the burning time of a 100-nm-size particle (Alex®) results in a lifetime of only approximately 160 and 920 nsec, respectively, for n =1.8 and n =1.5.
Experimental observations support the very short life computed for combustion of an Alex® particle that had originated from a solid propellant. Wang and co-work-ers[33- found that when Alex particles are flash-heated to the boiling point, 2740 K, in the presence of a nitrocellulose (NC) oxidizer, the energy release occurs in — 5 nsec even much faster than predicted by the Brooks and Beckstead model. Ivanov and Tepper[23- noted from highspeed cinematographic photographs of a burning propel-lant that the combustion of Alex® is complete at the burning surface, while micron-size aluminum is known to burn throughout the throat and often past the nozzle.
Alex® as an Additive to Hybrid Propellants
The classic hybrid technology uses LOX with an HTPB grain that contains either no oxidizer, or just enough to react with the HTPB so as to be a gas generator. This creates low molecular weight organics that are forced into the engine and then react with the liquid oxidizer. The hybrid fuel technology offers a number of advantages including the ability to throttle the engine in real time, stop and start and shift from combustion with on-board oxidizers to air. Should the mission require rapid launching, then the hybrid with storable oxidizers offers advantages over cryogenic fuels. Also, the grain case is smaller and much lighter without the heavy inorganic oxidizer that is in conventional solid propellants. Hybrid solid propellants are much easier, safer, and less costly to manufacture because the oxidizer is not combined into the grain. Also, there are fewer corrosion problems as the fuel is solid. These advantages would be very useful in a rocket-based combined-cycle (RBCC) engine.[34]
Hybrid problems include the fact that the distance of the flame to the burning surface is an order of magnitude larger than with solid propellant. This makes heat transfer an order of magnitude smaller and consequently the regression rate is an order of magnitude smaller. Because the main flow is rather stratified, the mixing process and therefore the combustion can be incomplete. To achieve complete mixing and combustion of oxidizer and fuel, an aft combustion chamber is added to the hybrid motor.
However, many of these problems can be ameliorated by adding Alex®. Chiaverini et al.[28] added 10 wt.% Alex® to an HTPB slab and found an increase of 70% in the regression rate. They also noted much smoother burning in the case of Alex®-loaded HTPB. Table 1 shows how using Alex® increases the specific impulse and density-specific impulse of a hybrid and compares it to NTO/Hydrazine.
Table 1 Specific impulse of aluminized HTPB resins with liquid oxygen (LOX)
 |
 |
 |
 |
 |
|
0% Al |
100% |
LOX |
371 |
392 |
|
HTPB |
|
|||
|
25% Al |
75% |
LOX |
376 |
423 |
|
HTPB |
||||
|
50% Al |
50% |
LOX |
370 |
458 |
|
HTPB |
||||
|
Hydrazine |
N2O4 |
362 |
442 |
Alex® as an Additive to Liquid Propellants
Palaszewski and Powell,[35- in some theoretical computations, demonstrated that 0-, 5-, and 55-wt.% aluminum loaded into RP-1 gel propellants provide benefits over neat RP-1. The 0-wt.% loading is attractive when safety over traditional RP-1 (ungelled) is desired and the 5-wt.% loading gave the maximum theoretical specific impulse. The 55-wt.% loading was chosen based on the fact that a liquid-based booster with O2/RP-1/Al could conform to the volume constraints of the current solid rocket booster even with potential two-phase flow losses taken into account. Palaszewski and Zakany[7,9] did some combustion experiments with these three mixes using micron-size aluminum for the 5% and 55% loadings. While relatively good performance was obtained, the engine efficiencies were not sufficient for NASA missions.
Mechanisms of Combustion of Gelled Aluminized Propellants
The combustion of a heterogeneous two-phase gel of liquid fuel and aluminum particles is essentially a two-step process. The first step is the combustion of the liquid droplet, which involves boiling of the liquid. A vapor blanket of fuel exists around the two-phase droplet and the flame zone is at some distance beyond the liquid-gas interface, protecting the aluminum from oxidation. At the temperatures and pressures that exist in the rocket engine, the aluminum will be molten. Aluminum combustion would be delayed until the hydrocarbon is burned away, and during that interval the micron-size aluminum droplets could coalesce. The smaller the hydrocarbon droplet the less time there is for the aluminum to agglomerate. The second step in the process involves the combustion of the now dry aluminum droplet. The mechanisms associated with this second step would be very similar to that occurring had the particles been generated from a solid propellant grain such as Al/organic binder/solid inorganic oxidizer. A nanosize aluminum particle would therefore be expected to have a life less than a microsecond, several orders of magnitude shorter than if micron-size aluminum had been dispersed into the kerosene.
Development and Formulation of Aluminized Gels
The authors were sponsored by NASA to evaluate the benefits of using Alex® in lieu of micron-size aluminum powder as a dispersed phase in RP-1.[36] Wetting and gelling agents were used as additives to aid in homoge-nization and providing dynamic stability. Fumed silica (Cab-O-Sil® grade M5) was used as a gellant and the stability of the gel was determined by centrifugation for 1 hr at 1300 rpm. It was found that when Alex® loading was greater than about 25 wt.%, fumed silica was unnecessary as the powder acts as a pseudo-gellant. A 30 wt.% Alex® in RP-1 gel was found to be dynamically stable when stored for 2 years at room temperature. A nonanionic surfactant, specifically Tween-85 (polyoxyethylene sorbi-tan trioleate), was used because it was more effective as a wetting and dispersing agent for the aluminum particles than anionic and cationic surfactants.
The viscosity of the gelled formulations was then measured so as to determine the practicality of pumping the mixture and spraying it into a combustion chamber. Spray characteristics of a given gas-liquid injector are a function of the liquid surface tension and the gas and liquid velocities, densities, and viscosities.[37] Liquid propellants typically have viscosities near 1 mPa sec and have Newtonian-type flow behavior.
Green and co-workers[37] reported absolute viscosity value of 1.89 mPa sec for RP-1. This value remains constant in a wide range of applied shear rates from 1 to 100,000 sec-1 Gelled propellants typically have viscosity in the range of 20 to 50 mPa sec at the shear rates in the range of 105 to 106 sec -1 that are typically encountered in engine injectors.[38] This is within the range of the pumping systems currently used in rocket engines. As the viscosity of a gelled fuel is a function of variables other than temperature (e.g., shear rate and temporal rheological effects such as thixotropy and gel relaxation time), the rheology is non-Newtonian, and viscometry is reported as ”apparent” viscosity at a specific shear rate.
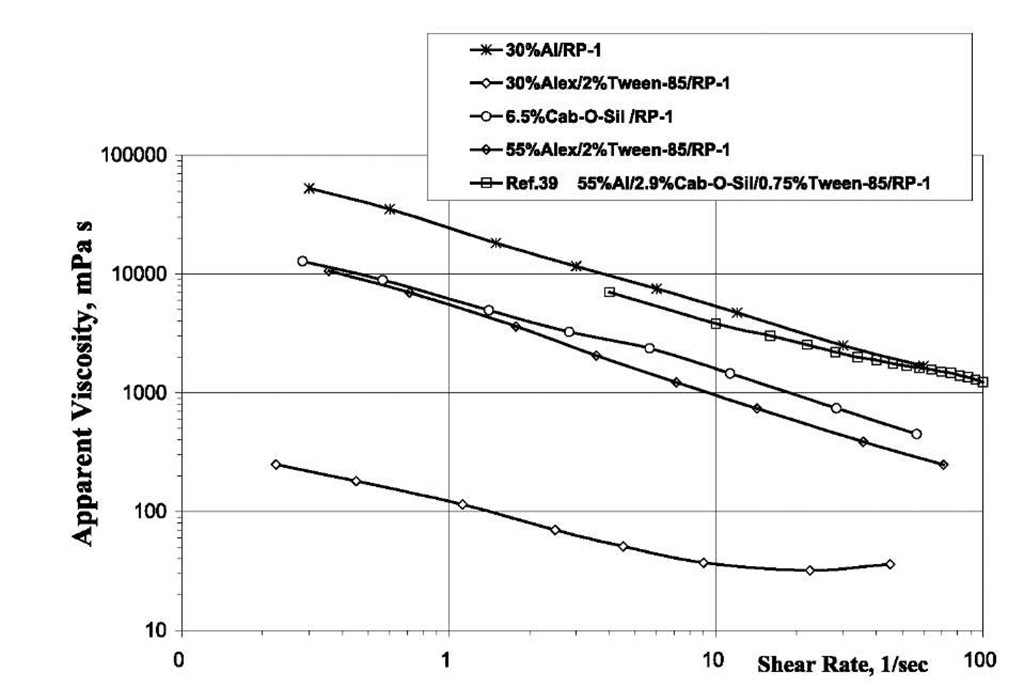
Fig. 2 shows the viscosity of Alexgel fluids and compares them with the viscosity measurements of Rapp[39] for micron-size aluminum/fumed silica/RP-1 gel permitting extrapolation to strains characteristic of the injector. Note the reduced viscosity when a surfactant is added to a 30% Alex®/RP-1. A power law rheological model (t=K-yn, where t is a shear stress, g is a shear rate, n is effective flow behavior index, and K is a constant of effective consistency) can be applied to model flow behavior. In general, power law is applicable to model flow behavior of pseudo-plastic fluids with zero yield point. Gelled fuels evaluated in Refs. [7], [9], [39], and [40- have very small yield values ranging from 0.03 to 22 Pa. These yield points are greatly exceeded in injector fluid flow, so their contributions are negligible. By extrapolation of viscosity data to high shear rates using a power law rheological model to the large shear rates encountered in engine injectors, the gels have viscosity near kerosene and therefore within the capability of the pumping systems currently used in rocket engines.
Fig. 2 Apparent viscosity of aluminized fuels.