INTRODUCTION
It is now widely recognized that the fundamental properties of materials strongly depend on the size of crystallites, especially if they are in the nanometer regime. Most of the physical, optical, and electronic properties of a bulk solid vary when the crystallites are in the nanoscale regime. If the grain size can be maintained at nanoscale dimensions, this creates major opportunities to design advanced materials with enhanced properties.
The emerging nanometals industry encompasses nano-particles, nanolayers, thin films, nanofibers, and bulk nano-structured metals and alloys. This topic focuses on metal nanoparticles, their properties and uses, with special emphasis on those manufactured by the electroexplosion of metal wire (EEW), a process that is the most commercialized and has produced the greatest diversity of metal nanopowders.
Nanometals are in the process of becoming one of the major feedstocks for a host of emerging technologies and industries. The body of knowledge on nanosize particles has grown throughout the latter part of the twentieth century as various processes for producing them were developed. With the exception of precious metals, most of the focus has been on ceramics and nonmetallic materials. More recently, there has been an increasing interest in other metallic nanoparticles. Handling them is problematic because they are highly reactive and difficult to produce, handle, and ship.
Nanosize precious metals have already had a long history of development and use as catalysts and in photography because of their chemical stability in water and air. Precious metal catalysts in a ceramic matrix, as in automotive catalysts, or dispersed homogeneously in media have had a profound affect on industrial processes. The increasing availability of other nanosize metals should lead to a plethora of new applications from composites to chemicals.
NANOMETAL PROCESSES
There are over a dozen companies that are currently developing processes for nano metal particles, but only a few companies routinely manufacture and supply them. Production quantities are currently limited to only grams or a few kilograms but some companies claim they are building facilities to produce tens to hundreds of kilograms per day. As a result of the small quantities, the prices range from several hundred to a few thousand dollars per kilogram. Most companies do not stock nano metal powders but will produce them on a custom basis.
The manufacturing processes for micron-scale powders, such as water atomization, are generally not adaptable to nanometer size metal powders, mostly because the powders would be oxidized in the environment in which they are produced. The methods currently being developed for nanosize particles include the following:
Ball milling—This method is capable of milling brittle materials to nanometer size, but fails for ductile metals such as copper unless milling is performed at cryogenic temperatures. Milling produces nonspherical particles with a broad particle size range. Dry milling of the more reactive metals can result in their contamination by air but wet milling under an organic fluid can protect the powder. Dry milling in an inert atmosphere is more complex and expensive. Precipitation from aqueous solution—Such processes are limited to precious metals because most other nanosize powders will react with water, causing surface contamination and, in the case of reactive metals, rapid and sometimes violent reaction. Metals that are precipitated from aqueous solutions generally tend to be porous and highly agglomerated. For example, silver particles can be produced[1] via the reduction of acidic silver salt solutions using aldehydes in the presence of silica sol, but the powder is highly agglomerated. The silica is subsequently hydrolyzed in caustic, and aided by a surfactant, it is separated from the agglomerated silver powder. Bonet et al.[2] describe a method for producing deagglomerated precious metal particles smaller than 10 nm by heating a mixture of precious metal salts with hot 150°C) ethylene glycol using polyvinylpyrrol-idone (PVP) to minimize agglomeration.
Organometallic synthesis in solution—This method employs the use of kinetically controlled reactions of organometallic precursors usually in nonaqueous solvents to product metal nanoparticles. The process can also utilize redox chemistry to reduce an inorganic precursor. The advantage of this process is that mono-disperse populations of small (< 50 nm) particles is possible, and the disadvantage is that Schlenk-type techniques may be necessary.
Evaporation/condensation under vacuum—Vacuum-driven thermal evaporation is limited to low-boiling-point metals such as aluminum and copper. Higher-boiling-point materials can be evaporated by e-beam or laser heating, but the capital and operating costs of such processes are high, favoring the development of processes that operate at or near ambient pressure.
Evaporation/condensation of the metal in an inert gas environment—Because most of the nanometals will react with oxygen or nitrogen to form oxides and nitrides, such processes require an inert gas cover. Generally, argon is used because of its lower cost compared to helium. Higher energy or impulse heating, such as laser bombardment or electric discharge, can attain higher temperatures and volatilize higher boiling metals compared to slower heating, e.g., via resistance heating of a crucible containing the molten metal. Ablation is well suited to refractory materials especially when employing laser-based heating. This has been performed both with excimer and CO2 lasers for a variety of high melting materials. Once evaporated, the metal nucleates in the cold inert gas and coalesces to larger particles during transport to the collector. Eifert and Gunther[3] describe a pilot-scale reactor using a cryogenically cooled condenser with a scraper to remove product from the condenser. Other collection approaches involve an electrostatic filter and cyclone separators.
Thermal decomposition of a salt or organometallic precursor in a flame or plasma-Axelbaum et al.[4] produced nanosize metal particles submerged in a sodium chloride shell by flame reaction of a metal salt such as titanium tetrachloride with sodium vapor in argon. The NaCl encapsulate is removed from the particles by both washing and subsequent sublimation at 800°C. There is potential for ionic contamination of the product, as well as sintering that could occur during sublimation of the salt.
The electroexploded wire (EEW) process—The EEW process is a physical process converting wire into nano particles in the absence of any chemical reaction that could contaminate the product. It is capable of producing a wide spectrum of nanopowders including high and low boiling metals as well as complex alloys. The EEW process has a very long history. In 1773, Edward Nairne applied an electrical pulse to a wire, causing it to explode in air to form aerosols of metal oxide. The EEW phenomenon also occurs at the moment of burnout of an incandescent filament, when a thinned filament is explosively converted to aerosolized powders in the burned out bulb, producing a momentary brilliant flash. The EEW principal is also used in exploding bridge wire (EBW) explosive detonators. There was a considerable amount of research carried out on EBWs in the 1940s and 1950s, showing that the pulse duration is only a few microseconds long and temperatures higher than 15,000 K are reached at the moment of explosion.
In the 1970s and 1980s, institutes of the Russian Academy of Sciences located in Tomsk, Siberia, invested considerable effort in developing the EEW process. Their innovation included adaptation of the process within an inert gas chamber and an effective mechanism for feeding wire from an internal spool to an electrode. Prior to 1995, most of their emphasis was for producing nano aluminum for energetics and nano copper for use in lubricating oil.
In 1994, Argonide Corporation was founded to commercialize EEW nanopowders. In 1997, a cooperative research and development agreement (CRADA) between the Department of Energy (DOE) and Argonide funded these Russian groups to further develop the process. Funds were also provided to the National Renewable Energy and Los Alamos National laboratories for characterization of the nano powders. With the exception of a 2-year halt during 1998-1999, the program continues to the present. The focus of this joint effort is on process improvement and applications development.
EEW powders can be produced from any metal that is available as ductile wire. Kilogram quantities have been produced with aluminum, copper, nickel, tin, indium, zinc, titanium, tungsten, niobium, tantalum, and silver and gram quantities with gold, palladium, and platinum. The alloys that have been produced include stainless steel, Hastelloy, and nickel-titanium.
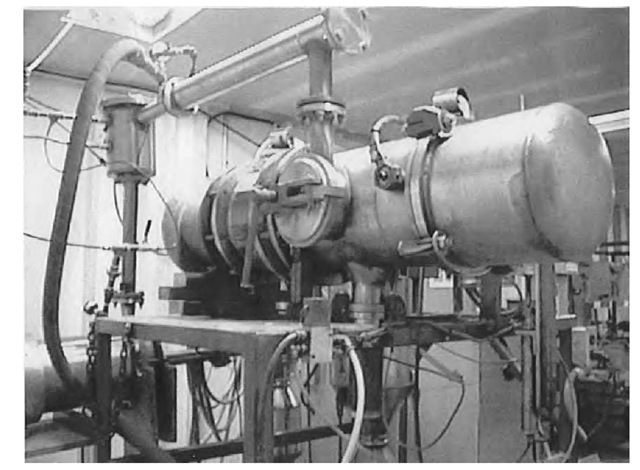
Fig. 1 shows an EEW machine, where the main reactor is seen in the upper center. The chamber and associated piping include either one or two cyclone separators (one is visible under the main chamber). A reel of wire is enclosed in the right side of the chamber and is then fed through an electrically insulated baffle.
Fig. 1 Electroexplosion machine.
When the wire contacts a strike plate located in the center of the chamber, the circuit is closed causing a large pulse (102-103 J in 1 msec) to flow through the wire, creating a plasma. A very strong field is formed that contains the plasma during the microsecond pulse. When the vapor pressure of the metal exceeds the ability of the field to contain the plasma, there is an interruption in current flow, and containment by the field is lost, allowing clusters of metal atoms to be projected at supersonic speed through the argon. The metal clusters are less than about 10 nm, but coalescence occurs as flowing argon (at 2-3 atm) transports the clusters to a cyclone separator, where the now agglomerated particles are collected. At this point, further growth and coalescence is frozen, producing spherical particles with an average particle size of 40-150 nm, depending on the wire size, exploding regime, and freezing point of the metal. X-ray diffraction shows the powders to be relatively pure elemental metal.
Several hundred grams of powder per hour are produced in a single exploder with the rate proportional to the specific gravity of the metal. The energy density introduced in an exploding wire is one of the most important parameters in the process. Pulse energy is optimized by balancing the energy input with the diameter of the wire to produce electrical energy equivalent to the sublimation energy of the metal. With the current power supplies, the best trade-off between the particle size and production rate is achieved by using 0.3-mm diameter wire and results in nanopowders with an average size of 100 nm. Smaller particles (down to about 50 nm average particle size) are produced by substantial reduction in wire diameter, but at the cost of reduced throughput of metal powders. Below a critical energy density, no explosion will occur and the wire is evaporated leading to coarser particles.
Particle size can also be reduced by a higher rate of electrical explosions and by reducing the overpressure of inert gas. Low levels of active gases, such as nitrogen and oxygen, if added to the argon in the reactor, often result in reduced particle size without much contamination of the metal. The apparent mechanism is the formation of fine oxide coatings such as aluminum oxide on aluminum, inhibiting coalescence of the metal particles. Excessive oxygen will result in the production of nanosize metal oxides such as (gamma) aluminum and titanium oxides, with particle size about 30-50 nm in diameter.
Powder is collected in the separators until they contain about 1 kg. The collector is frequently emptied into a collection drum. The EEW process is scalable, and a newer reactor design increases the throughput of the reactor and minimizes downtime. In the newer device, the explosion frequency has been increased from about 0.5 to 3 Hz, as recirculation of the argon gas is increased. A feed-through system was developed so that a large spool of wire can be fed into the reactor without opening the chamber and interrupting the process. Also, the arrangement and length of the piping was altered to improve sedimentation, particularly of occasional large particles that are produced by the conventional process.
The EEW process is limited to particles with average sizes less than about 300 nm. Particles as small as 20 nm have been made in experimental quantities. Virtually all the metals produced by this process are combustible, and several of them, such as aluminum, iron, titanium, and zirconium, are either pyrophoric or nearly so. The powders are collected and protected from oxidation by the argon in the reactor. The more reactive powders are transferred to liquid hydrocarbon. In the case of Alex® nanoaluminum, the particles are passivated by adding dry air before removing it from the chamber and are then packaged as a dry powder.
All EEW powders are handled and shipped as hazardous (combustible) metal powder. Alex® is the nanometal made in the greatest quantity, followed by nickel, copper, tungsten, stainless steel, silver, and zinc. While Ni, Cu, and W may oxidize when exposed to air, they are relatively well behaved compared to dry iron, stainless steel, or titanium powders. The preferred method of packaging dry metal powders is in glass ampoules, and they are offered in quantities of at least 100 g net weight of powder.
The principal advantages of the EEW process are:
1. As with gas condensation techniques, contamination by chemical reactants or by reaction with solvents is avoided.
2. Spherical particles are ordinarily produced.
3. As compared to other high-temperature processes, virtually all of the electrical energy in the EEW process is directly converted to heat. There is little opportunity for convection or radiation heat loss during the very rapid 1 msec) pulse.
4. Alloys can be converted to nanopowder with no measurable segregation as compared to evaporative processes, where the composition of the particles will vary.
5. The process operates with pressurized inert gas, simplifying the weight and cost of the reactor and piping.
Alloys are produced on a custom basis, so long as the fine wire is either commercially available in a continuous filament or can be produced with sufficient ductility to be fed into the wire feed mechanism without breakage.
PHYSICAL AND CHEMICAL PROPERTIES
Table 1 summarizes the surface area and crystallographic character of several EEW powders including aluminum oxide (g phase) and aluminum nitride. As with other nano powders, the principal difference in the physical and chemical properties of nanometals results from their greater surface area as compared to fine (micron) size particles. The surface area is inversely proportional to the square of the diameter of the particle, so a 100-nm spherical particle has a surface 1000 times greater than a 10-mm particle. This creates opportunity for creating materials with new and useful properties, but also poses difficulty in handling, shipping, and storage.
Table 1 Electroexploded nanopowders and their characteristics
|
Particle size |
Average particle |
Metal content |
Surface area |
Particle |
|
|
|
Powder |
distribution function |
size [nm] |
[%] |
[m2/g] |
morphology |
Crystalline defects |
|
Al |
Normal-logarithmic |
80-150 |
Passivated > 92 |
8-18 |
Spherical |
Numerous defects |
|
Al |
Normal Gaussian |
30-50 |
Depends on particle size distribution |
20-48 |
Crystallites |
No information |
|
Cu |
Normal logarithmic |
100-150 |
passivated > 90 nonpassivated > 96 |
5-8 |
Spherical |
Numerous defects |
|
Cu |
Normal Gaussian |
30-50 |
passivated > 90 nonpassivated > 96 |
~ 12 |
Crystallite faces |
Small amount of defects |
|
Ni |
Normal logarithmic |
80-100 |
passivated > 95 nonpassivated ~ 99 |
4.5-6 |
Spherical |
Numerous defects |
|
Ni |
Normal Gaussian |
30-50 |
passivated > 95 nonpassivated ~ 99 |
~ 7.5 |
Crystallite faces |
Small amount of defects |
|
Zn |
Normal logarithmic |
100-200 |
passivated > 90 |
4.1-6 |
Spherical |
Numerous defects |
|
g-Al2O3 |
Normal Gaussian |
30-50 |
Not applicable |
20-45 |
Spherical |
Small amount of defects |
|
AlN |
Normal Gaussian |
50-60 |
Not applicable |
~ 36 |
Crystallite faces |
Small amount of defects |
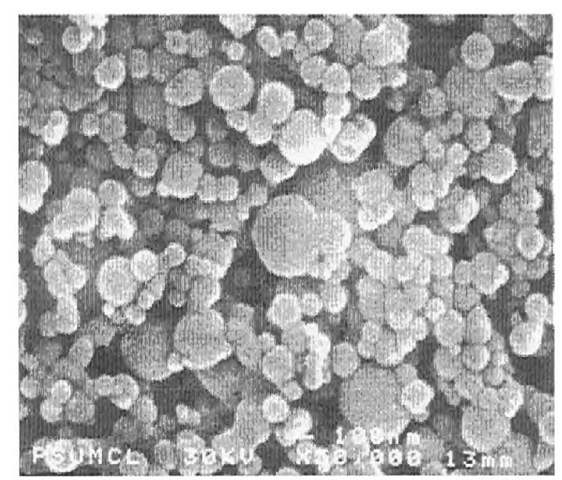
A scanning electron microscopy photo of Alex® nanopowder is seen in Fig. 2. Surface area [Brunauer-Emmett-Teller (BET)] measurement is generally a good surrogate for determining average particle size because EEW powders are mostly spherical, fully dense, and smooth. The surface area of Alex® with 100 nm average particle size ranges from about 10 to 20 m2/g. Fig. 3 shows a sector of an Alex® particle with a passivation coating about 2.5-3 nm thick. X-ray powder diffraction shows these particles are primarily metallic aluminum, while the coating is principally aluminum oxide with minor amounts of nitride and an oxynitride. The amount of active aluminum for a 100-nm size average particle is approximately 88-90 wt.%. The oxide content rises with diminishing particle size, as the 3-nm oxide layer comprises an increasingly greater mass.
The thermal properties [differential thermal analysis (DTA)] of Alex® powder when heated in air, oxygen, and nitrogen was compared[5] to that of micron size aluminum. Alex® powder reacts rapidly with these gases, producing very sharp exotherms that occur well below the melting point (660°C) of aluminum, while 20-p.m-size aluminum does not react until about 1000°C.
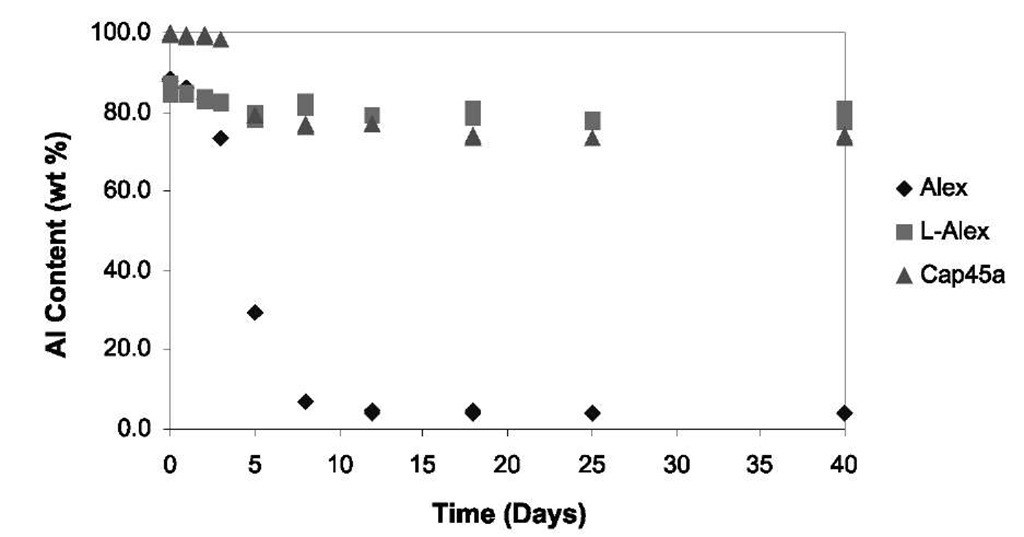
An organic coating has been developed for many of the nanometals and is being used for coating aluminum and other reactive nanometals. The coating (called L-Alex®) is based on the reaction of a carboxylic acid (palmitic) acid with the aluminum powder rather than coating it with oxide. The resistance of L-Alex® to moisture attack during accelerated aging was com-pared[6] to oxide-coated Alex® and to a 17-p.m-size aluminum powder (Cap45a). The test involved exposure of a thin layer of the aluminum particles in a dish within a temperature humidity chamber. Each day, a sample of each powder was removed and titrated for residual aluminum metal. The powder in the dish was mixed daily to expose fresh surface. Aging was performed at several temperatures and humidity levels from room temperature and dry conditions to a maximum of 60°C/75% relative humidity (RH). Fig. 4 shows a 20% and 70% aluminum metal loss, respectively, in the case of Cap45a and Alex® powders, but the L-Alex® showed little aluminum loss throughout the 40-day test exposure. The study also showed that bayerite (Al(OH)3) is the major product of hydrolysis rather than Al2O3. Degradation of the Cap45a powder ceased after day 12 when exposed to harsher conditions as a result of the buildup of a layer of bayerite.
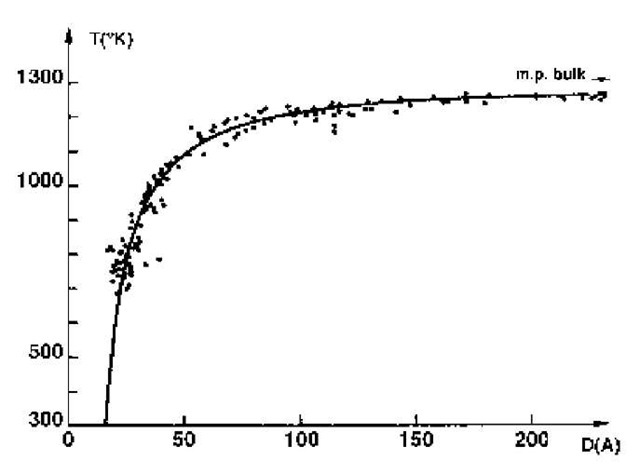
Melting point is one of the properties that can be altered by reducing particle size down into the nanometer range. Fig. 5 shows the melting point of gold[7] as a function of particle size. Melting point reduction is not really significant until the particle size is less than about 10 nm.
Fig. 2 Field emission electron microscopic view of Alex® (50,000 x).
Fig. 3 High-resolution (400,000 x) transmission electron microscope of alumina layer on the surface of spherical Alex® particle.
EEW powders have crystal defects, faults, and twins caused by rapid quenching during the process. Fig. 6 shows a high-resolution transmission electron microscope (TEM) view of EEW nickel showing twins and polytwins. The clusters are propelled by electroexplosion at 2 km/sec through the cold argon, which results in a quenching rate of about 108 °C/sec. The disorder in the EEW aluminum crystal extends into the oxide outer layer, causing the powders to be physically metastable and more chemically reactive. Fig. 7 is a DTA of EEW silver showing an exotherm at about 220°C, far below the melting point (960°C) of silver. The internal energy is estimated to be about 40% of the heat of fusion. When alloy wire is electroexploded, the very high quench rates often produces nonequilibrium phases. For example, EEW 300-stainless steel can be heterogeneous, containing not only the expected austenite phase, but also alpha iron, nickel, and others components in the Fe-Ni-Cr phase diagram.
Fig. 4 Aluminum powder accelerated aging in 60°C/75% RH air.
Fig. 5 Melting point of gold particles as a function of particle size.