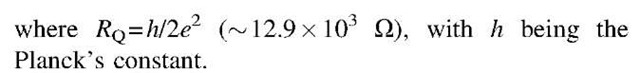INTRODUCTION
Nanotechnology is a catchword that evokes excitement in researchers and captures the imagination of laymen. The excitement in the research community stems from the fact that theoretically predicted variations in the physical properties of solid objects as their dimensions approach a few nanometers (1 nm= 10"9 m) —viz. lowering of melting point, Coulomb charging, novel magnetic, optical phenomena, etc.—have been verified experimentally.1-1-4-1 This experimental verification has been possible because of rapid strides made over the last two decades, both in techniques for synthesis and in tools for characterization of individual nanoscale objects. These advances have also opened up a vast array of potential technological applications and have occurred just as the limits of photolithography-based solid-state technology are being reached. Coupled with the revolutionary impact of solid-state electronic devices on our lives, advances in synthesis, manipulation, and characterization of nanomaterials have made nanotechnology—and in particular nanoelectro-nics—a cynosure of public interest.
There are two alternative approaches for fabricating nanoelectronic devices: the ”top-down” and the ”bottom-up” approaches. The top-down approach is similar to current photolithographic techniques used to produce microelectronic devices. It consists of ”chiseling” nanometer-scale features in bulk materials. Using such techniques as e-beam lithography and x-ray phase shift lithography, this approach can now produce nanoscale features (<50 nm) and has the decided advantage of being compatible with current microelectronic processing methods and design concepts. However, this approach suffers from two important drawbacks: 1) processing costs rise exponentially as feature size decreases; and 2) the surfaces and interfaces produced by this approach exhibit atomic-scale imperfections, which critically degrade device performance as feature size approaches nanometer dimensions. The bottom-up approach consists of ”building” the device or circuit by assembling it from preformed nanoscale ”bricks.” Bottom-up processing, involving the serial manipulation of nanoscale objects, is technologically impractical. However, it is often possible to induce nanoscale objects to assemble themselves into desired structures. It is such biologically inspired self-assembly which holds the greatest promise.
There are a number of interesting nanoelectronic building blocks, viz. metal nanocrystals and nanowires (both magnetic and nonmagnetic), semiconductor nano-crystals and nanowires, and carbon nanotubes. In this topic, we focus solely on nonmagnetic metal nano-crystals. Two characteristics of metal nanoparticles are critical for assessing their usefulness in nanoelectronic applications: 1) the ease with which bare metal particles cold weld on contact to form hard aggregates; and 2) the tendency of metal particles to oxidize in an atmospheric environment especially in the presence of water molecules. The first characteristic means that the surface of a metal nanoparticle must be passivated by attachment of a monolayer of capping ligands or surfactant molecules before any attempt is made to assemble these particles into a uniform nanostructure. We will refer to such encapsulated particles as molecularly protected nanopar-ticles (MPNs). The second characteristic means that only noble metals such as Au and Ag will form nanoparticles that are not rapidly oxidized when exposed to an atmospheric environment. Linear alkanethiol molecules readily react in solution with both Au and Ag nanopar-ticles to form stable MPNs. These MPNs can be manipulated as stable physical species in a variety of organic solvents. The ability to manipulate Au and Ag MPNs in organic liquids, to synthesize macroscopic quantities of these particles with diameters in the 220 nm range, and to control the interparticle spacing in arrays of these particles by changing the length of the alkanethiol molecules coating the metal core make Au and Ag MPNs ideal building blocks for the self-assembly of nanoelectronic devices. To date, most studies of metal MPNs are of Au nanoparticles encapsulated by an al-kanethiol monolayer.
ELECTRONIC APPLICATIONS OF METAL NANOPARTICLES
The simplest metal nanoparticle-based electronic material is fabricated by suspending Au MPNs in an organic solvent such as toluene, casting or spraying this suspension on a solid substrate, and allowing the solvent to evaporate. The nanoparticles agglomerate as the solvent evaporates to form a loosely aggregated solid. If the original suspension is dilute and care is taken to allow the solvent to evaporate slowly, small 3-D superlattice domains or ”ordered MPN crystals” form as the suspension becomes supersaturated; however, in general, the solid phase that forms is amorphous. The closest interparticle spacing in the solid is determined by the length of the alkanethiol molecules encapsulating the Au particles. Because this spacing is on the order of a nanometer, these amorphous solids are weak electrical conductors in which electrons tunnel through the organic layers separating the Au particles and hop from particle to particle. Snow and Wohltjen[5] have measured the electrical conductivity of amorphous films of Au MPNs as a function of the ratio of the diameter of the gold core to the length of the alkanethiol encapsulant. They measured electrical conductivities ranging from 10-6 to 10-12 (O cm)-1 and found that the conductivity at a given temperature increases as this ratio is increased. They have also shown that, when such an MPN solid is exposed to volatile organic vapors, its electrical conductivity is a function of the partial pressure of the organic molecule.[6] This effect is most probably because of the absorption of the organic molecule into the alkanethiol layer surrounding the gold nanoparticles. Quantum tunneling varies exponentially with distance, and a small swelling of this layer results in a large variation in tunnel resistance. Thus even as simple a structure as an amorphous solid of Au MPNs constitutes an interesting electronic material, and a film of this nanostructured material can act as a chemi-resitive sensor. Unfortunately, films of alkanethiol-coated Au nanoparticles are not strongly species-selective.[6] However, adding different chemical functionalities to the ligand shell around the Au particles may be a way to increase the chemselectivity of the film.
Amorphous films of Au MPNs can also be used to fabricate low-resistance electrical conductors on flexible substrates. Low-resistance conductors are important components of high-Q inductors, capacitors, tuned circuits, and interconnects. An inexpensive method for fabricating such conductors on flexible substrates is critical for the development of ultralow-cost microelectronic systems such as radiofrequency identification tags. Using a dense suspension of Au MPNs in a volatile organic liquid, it is possible to print micron-thick lines via either ink jet or silk screen techniques on a substrate. If the Au particle diameter and the alkanethiol encapsulant are optimized, these low-conductivity Au MPN lines can be converted into high-conductivity Au lines. This transformation is made possible by the low melting temperature and low shear resistance of Au nanoparticles.[2,7,8] It is accomplished either by thermally annealing the lines at a relatively low temperature (<150°C),[9] or by exposing them to laser radiation.[10]
Although the electrical conduction behavior of an amorphous film of Au MPNs has technological potential, placing a single Au MPN between two electrodes provides a much richer range of electronic behavior. In this configuration (Fig. 1), the nanoparticle is able to suppress all electrical conduction at low-bias voltages. This phenomenon is called ”Coulomb blockade.” Coulomb blockade occurs when the electrostatic energy increase caused by adding a single electron on a capacitatively coupled metal island is much larger than the thermal energy of the electrons:
where e is the charge on an electron, C is the effective capacitance of the metal island, kB is the Boltzmann constant, and T is the absolute temperature of the metal island. In the case of metal MPNs, the capacitance C is directly proportional to the radius of the metal nanopar-ticle. For room temperature operation, Eq. 1 is satisfied when the particle diameter is less than approximately 23 nm. Larger particles are able to suppress all electron transport only if their temperature is lowered. For Coulomb blockade to be observed, the tunneling resistance (R) to and from the metal island must also be much greater than the resistance quantum (Rq):
Fig. 1 (a) Schematic representation and equivalent circuit of nanostructure consisting of a single metal MPN placed between two electrodes. (b) Ideal I-V curve for this nanostructure illustrating Coulomb blockade at low bias and Coulomb staircase as bias is increased.
When both Eqs. 1 and 2 are satisfied, the I-V curve for an asymmetric junction {Rr^RL or RL^Rr} shows characteristic steps in the current, called ”Coulomb staircase” (Fig. 1). The asymmetry in the resistance means that the flow of electrons is controlled either at the right or left tunnel junction, and so electrons tend to accumulate on the metal island. Because of the electrostatic energy associated with the charging of the island, an additional electron is not added at steady state until this excess energy is compensated for by increasing the external bias. Both Coulomb blockade and Coulomb staircase phenomena have been experimentally verified at room temperature using an Au MPN.[3,11] In this experiment, a scanning tunneling microscope (STM) was used to measure the I-V characteristics of a vertical nano-structure, fabricated by depositing a single 2-nm-diameter gold particle on a gold substrate that had been coated with a self-assembled monolayer (SAM) of a double-ended thiol molecule.
The structure shown in Fig. 1 is the simplest example of a large class of nanoelectronic logic and data storage devices that are based on the controllable transfer of single electrons between small conducting islands separated by tunnel barriers.[12] For example, Tucker[13] proposed a nanoscale field effect transistor (FET) based on modulating the voltage range of the Coulomb blockade by applying an asymmetric bias on a conducting island transverse to the direction of current propagation. Fabrication of such a single electron tunneling-field effect transistor (SET-FET), using metal nanoparticles, requires the ability to place 1-D chains of metal MPNs on an insulating substrate with variable interparticle spacing and the ability to address a nanoparticle having a diameter of ~2 nm from three directions. At present, there does not appear to be any way to self-assemble such a device. Although, in principle, any SET architecture can be constructed using metal MPNs, the technical difficulties involved in self-assembling complex SET circuits that can operate at room temperature seem overwhelming.
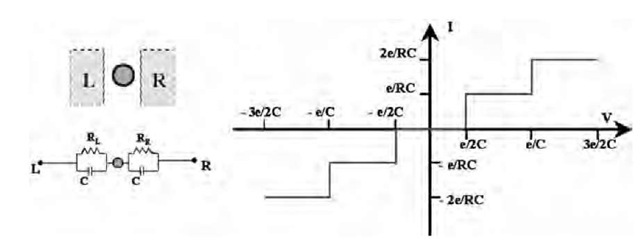
Roychowdhury et al.[14] have suggested a logic architecture (Fig. 2) involving metal MPNs that does not require room temperature Coulomb blockade for its operation. This architecture consists of a uniform 2-D array of Au MPNs with input and output addressing along the edges of the array. The nanoparticles are coupled through electron tunneling barriers both to each other and to a semiconductor substrate that is a resonant tunneling diode (RTD) structure. The computation is performed by allowing the array to relax to its ground state, which depends on the Coulomb interactions of adjacent islands for a particular set of input voltages. This 2-D array architecture is compatible with the self-assembly and microcontact printing techniques described later in the present topic, and recent reports on the formation of 8-nm-wide metallic lines[15] and addressable cantilever arrays[16] suggest that edge addressing issues and even readout of the charge state of individual nanoparticles may be tractable.
The weak interaction forces that exist between metal MPNs, although ideal for promoting self-assembly from solution, are not strong enough to produce 1-D, 2-D, or 3-D arrays that are structurally robust. Thus it is often necessary to strengthen the self-assembled structures formed by Au MPNs. This can be accomplished by displacing the monofunctional alkanethiol molecules coating the particles with difunctional molecules that bind the particles to each other or to the substrate. Both dithiol molecules and diisonitrile molecules are able to displace monothiol molecules from Au.[17,18] Attaching conjugated molecules having distributed electronic states in the gap between adjacent metal nanoparticles in an array, or between a metal nanoparticle and a substrate, is also an attractive way to self-assemble another kind of electronic nanostructure. The metal-molecule-metal bridge that is established in this manner is the focus of the emerging field of molecular electronics.[19,20] The diode structure shown in Fig. 1 can now be thought of as being replaced by one in which the conjugated linking molecule takes the place of the Au MPN. To date, experimental measurements of electron transport through such metal-molecule-metal structures have been attempted either by introducing a ”nano” gap between two metal electrodes and adsorbing the desired organic molecules on the electrodes, or by STM manipulation of metal atoms and a single organic molecule on a substrate. An elegant example of this latter type of experiment is reported in the recent paper by Nazin et al.[21] Although interesting electronic behavior has been demonstrated,1-22-1 the problem of how to self-assemble logic or memory circuits is still unsolved. A fruitful approach to this problem may be to self-assemble molecular electronic devices using ”linked arrays” of metal MPNs.
Fig. 2 Schematic of nanoelectronic architecture proposed by Roychowdhury et al. for Boolean logic. Nanostructure consists of an ordered 2-D array of Au MPNs linked to a RTD substrate.
Au MPNs have been used to fabricate lateral structures that are linked by conjugated molecules. Andres et al.[17] fabricated a linked monolayer of Au nanoparticles in the gap between lithographically defined electrodes by first self-assembling a uniform superlattice array of Au MPNs and then by replacing the alkanethiol molecules coating the Au particles with difunctional conjugated molecules. They measured electron transport in this monolayer film. Datta et al.[23] have proposed the use of quasi-1-D conductive ribbons of linked metal nanoparticles, which they term ”molecular ribbons,” as interconnects for semiconductor devices. It is difficult to fabricate linked mono-layer films that are free of defects when the length of the linking molecule does not closely match the spacing in the original MPN array. One way to solve this problem is to fabricate a linked bilayer film, such as that shown in Fig. 3.[24] This bilayer consists of two monolayers of Au MPNs with a molecular interconnect covalently linking the two layers. This nanostructure has been proposed as the basis of a novel chemiresistive sensor.[24] It is hypothesized that target molecules that ligate with the linking molecules will shift the electronic energy levels of the linking molecules, thereby changing the tunnel resistance between the two layers and the electrical conductivity of the film. If this hypothesis is confirmed, this scheme would provide a generic method for fabricating chemi-selective sensing elements.
Fig. 3 Schematic representation of a chemiresistive sensor element proposed by Santhanam. (a) Plane view and (b) cross-sectional view. Nanostructure consists of a uniform bilayer of Au MPNs in which the two layers are interconnected by adsorbate-specific organic molecules. This bilayer forms the channel of a electrical diode.
Au MPNs have also been used to construct linked vertical structures. They have been utilized as nanoscale contacts on a semiconductor substrate[25] and as nanoscale switches on top of redox molecules.[26] Such linked vertical structures represent an attractive paradigm for molecular electronics-based devices.
Metal MPNs are also being studied for hybrid microelectronic applications such as floating gate memory cells and multiple tunnel junction devices.[27,28] Metal nanoparticles provide an attractive choice for these applications because of the abundance of electrons near the Fermi level and the ability to tune their work function by changing the metal.
MPN SYNTHESIS
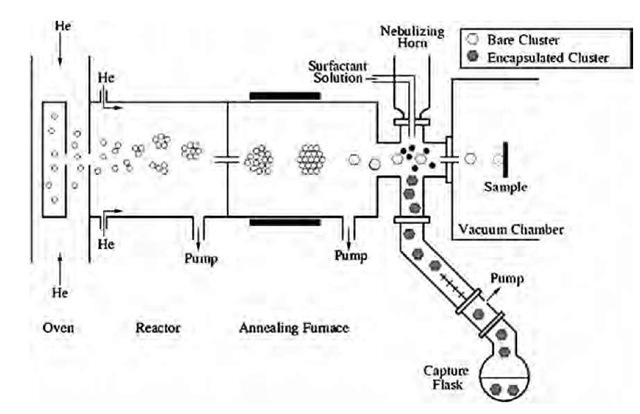
Metal MPNs can be synthesized either in the gas phase or in liquid solution. In either case, the essential requirement for synthesizing particles with a narrow size distribution is to initiate a temporally discrete homogeneous nucle-ation event followed by a slow growth regime, and to prevent particle aggregation. An apparatus for gas-phase synthesis of metal nanoparticles is shown schematically in Fig. 4.[29,30] Metal atoms, evaporated from a single crucible or a series of crucibles located in a resistively heated oven, are entrained in helium and induced to condense by mixing the hot flow from the oven with a room temperature stream of helium. Controlling conditions in the oven and helium flows controls the mean particle size. The particles are thermally annealed in the gas phase by passing the aerosol mixture through a tube furnace. They are scrubbed from the gas phase by contact with a mist of organic solvent containing surfactant molecules and collected as a stable colloidal suspension. The reactor shown in Fig. 4 also has provision for expanding a portion of the aerosol stream into a vacuum chamber to form a particle beam. Potential advantages of gas-phase synthesis are: 1) the ability to synthesize extremely small particles and to vary the mean particle size over a wide range; 2) the ability to produce pure metal particles, which may then be encapsulated with a wide variety of surfactants; 3) the ability to produce mixed metal particles even when the constituent metals are immiscible at room temperature; and 4) the ability to thermally anneal metal particles before encapsulating them with an organic surfactant.
Solution-based techniques for the synthesis of metal nanoparticles are based on the reduction of positively charged metal ions or ion complexes in solution usually in the presence of a capping ligand to arrest particle growth at a desired size. The major advantage of solution synthesis is the simplicity of the required equipment. The major disadvantage is the need for removal of excess reactants and for isolation of the particles as pure MPNs. In the case of Au particles, encapsulation by alkanethiols simplifies the isolation and purification steps greatly. A general technique for solution synthesis of nanoparticles with a controlled size and a narrow size distribution is to isolate small volumes of aqueous solution in which particle growth takes place by means of nonionic inverse micelles.[31,32] However, two homogeneous solution methods are more commonly used for the production of Au MPNs. The first method is reported by Brust et al.[33] This is a two-phase (water-toluene) reduction of AuCl4" by sodium borohydride in the presence of an alkanethiol. The Au ions are transferred to the toluene phase using tetraoctylammonium bromide, where they are simultaneously reduced by borohydride and capped by the alkanethiol. Once encapsulated Au particles have formed, they are precipitated from the toluene solution by the addition of a polar solvent, and then washed, dried, and resuspended in a nonpolar solvent such as toluene, hexane, or chloroform. The second method is reported by Giersig and Mulvaney.[34] This method makes use of the classical method of Turkevich et al.,[35] which produces charge-stabilized Au particles by reduction of AuCU" in water using trisodium citrate as the reducing agent. An alkanethiol dissolved in tetrahydrofuran (THF) is then added to this aqueous solution, causing encapsulation of the Au particles and their gradual flocculation. The capped Au particles can be extracted into cyclohex-ane. They are precipitated from cyclohexane by the addition of a polar solvent, and then washed, dried, and resuspended in a nonpolar solvent. Au MPNs produced by the Giersig method retain a residual charge before they are extracted into cyclohexane and can be deposited on a conducting substrate by electrophoresis.[34] After they have been washed, dried, and resuspended in a nonpolar solvent, there does not seem to be any difference between Au MPNs produced by the two methods; however, the Brust method appears to be more suitable for the synthesis of smaller particles (<5 nm) and the Giersig method appears to be more suitable for the synthesis of larger particles (>5 nm).
Fig. 4 Schematic representation of an aerosol reactor used to produce metal MPNs with a narrow size distribution. Metal atoms are condensed in a helium flow to form bare metal clusters (nanoparticles). The aerosol containing the nanoparticles then passes through an annealing furnace, after which the particles are encapsulated with an appropriate surfactant molecule and captured as a colloidal suspension.
The standard deviation of the diameter distribution of particles synthesized by either the Brust method or the Giersig method is about 10%. The size distribution can be narrowed by fractional crystallization.[36] This involves selective precipitation of the particles in a solvent mixture containing a good solvent and a poor solvent such as toluene/acetone. Whetten et al.[37] have used selective precipitation in the presence of excess alkanethiol surfactants to refine the size distribution of Au MPNs synthesized by the Brust method. Starting with Au MPNs having a mean diameter in the 1- to 2-nm range, they were able to prepare monodispersed samples having a fixed number of gold atoms. Stoeva et al.[38] have found that it is possible to refine both the size distribution and faceting of Au MPNs simply by heating a concentrated suspension of the particles in a process they have termed ”digestive ripening.”
Au MPNs produced by solution synthesis are compact, nearly spherical particles with a narrow size distribution that can be improved by size-selective precipitation; however, the gold cores are not necessarily faceted single crystals. Whetten et al.[37] report that in the 1.5- to 3.5-nm range, Au MPNs synthesized by the Brust method have faceted gold cores that are primarily single crystals with a face-centered cubic (FCC) lattice and a lattice constant close to the bulk gold value of 0.409 nm. High-resolution transmission electron microscopy (HRTEM) analysis of Au MPNs that were synthesized by the Giersig method with diameters in the 5- to 20-nm range reveals that these particles are primarily polycrystalline.[39] This is also true for Au MPNs with diameters greater than a few nanometers that are synthesized by gas-phase condensation, unless they are thermally annealed by heating them above their melting temperature and then allowing them to recrystallize in the gas phase. Then they become predominately single crystals or singly twinned crystals with an FCC lattice and a lattice constant of 0.409 nm.[24,39]
The ultimate Au MPN as far as controlled size and crystal structure are concerned is the triphosphine ligand-protected 55-gold-atom particle synthesized by Schmid et al.[40] almost two decades ago. These particles have an icosahedral geometry and exhibit Coulomb blockade behavior at room temperature. They played an important role in establishing the quantum size behavior of metal MPNs and in suggesting possible electronic applications of encapsulated metal nanoparticles.[41,42] However, the complex synthesis of these particles, which requires anaerobic conditions and diborane as a reducing agent, and the difficulty of replacing the triphosphine ligands with other ligands have led to these particles being studied much less than alkanethiol-encapsulated Au particles. A more convenient synthesis technique and schemes for utilizing exchange reactions to modify the characteristics of these particles have been reported.[43]
Because of their simplicity and flexibility, the solution-based synthesis routes of Brust et al.[33] and Giersig and Mulvaney[34] are currently the methods of choice for preparing Au MPNs for electronic applications. Similar solution-based methods are available for preparing semiconductor and magnetic metal MPNs.[36,44] Methods utilizing safer solvents and biosynthetic routes are being explored to address environmental concerns.[45,46]
FABRICATION OF ORDERED MPN ARRAYS
The ability to assemble nanometer-scale metal islands or particles into ordered arrays is a prerequisite for the successful fabrication of nanoscale electronic devices based on these building blocks. In what follows, we focus on self-assembly methods involving metal MPNs. ”Top-down” approaches using resist-based lithographic patterning will not be discussed at all. However, two methods that do not make use of metal MPNs are worth mentioning. The first technique is to self-assemble an ordered monolayer of polystyrene or silica spheres on a substrate and thermally evaporate metal atoms onto this colloidal mask. This results in nanometer-scale metal deposits forming on the areas of the substrate not masked by the colloidal spheres.[47] The second technique involves synthesis of metal nanoparticles inside structures formed using diblock polymers.[48,49] The major drawbacks of both of these schemes are that the metal islands or particles have largely uncontrolled shapes and typically have interparticle spacings much larger than molecular dimensions. Because of the ease by which Au nanopar-ticles can be thermally annealed, the first of these drawbacks may not be severe in the case of Au. Nevertheless, the large interparticle spacings make it difficult to see how electronic circuits can be fabricated using these methods. Self-assembly of MPNs that have been preselected as to size, shape, and surface chemistry into ordered arrays seems to be the most promising approach for fabricating nanoscale electronic devices.