INTRODUCTION
Metal clusters on oxides play a profound role in modern-day society because such systems are the basic ingredients in catalysts used for environmental protection, refining of fossil fuels, and production of chemicals. In the automotive three-way catalytic converter, for example, chemical reactions take place on nanometer-scale metal particles of Rh, Pt, and/or Pd distributed on an oxide of a high surface area, typically alumina.
Catalysis has thus been the main motivation for research on metal clusters on oxides. The improved ability to control the size and distribution of metal clusters coupled with the development of techniques for better characterization of the structural, electronic and chemical properties of metal clusters on oxides (primarily scanning probe techniques) has spurred an increased research activity in the field. Consequently, a series of interesting new results have been obtained, which have demonstrated how, e.g., the properties of the metal clusters may change in the nanometer-scale regime due to quantum size effects.
Atomic-scale investigations of industrial catalysts are hampered by the complexity of the systems. In this article emphasis will therefore be on metal clusters on oxides prepared and investigated in laboratory research environments, i.e., under ultrahigh vacuum (UHV) conditions. The advantage of such model systems is that they lend themselves much more readily to investigations based on a large variety of surface science tools. The article is intended to give the reader an overview of the techniques for synthesis and characterization of model systems, as well as to address important issues that are relevant for metal clusters on oxides in general. For further reading, reference is given to a number of reviews on metals on oxides.[1-5]
SUBSTRATE PREPARATION, GROWTH, AND CLUSTER MORPHOLOGY
Fig. 1 shows an image of a ruthenium catalyst for ammonia synthesis recorded with high-resolution transmission electron microscopy (HRTEM).[6] The Ru metal clusters appear as dark, almost circular regions on a lighter background of MgAl2O4. The experimental challenge of investigating (at the atomic level) systems of a structural complexity such as that in Fig. 1 is still so formidable that strong efforts are made to derive information from structurally simpler systems. In the following, focus will be on techniques for creating model systems of metal clusters on oxides.
Substrate Preparation
Typically, the metal clusters are supported on low-index surfaces of crystalline metal or semiconductor oxides, such as MgO, Al2O3, TiO2, Fe2O3, ZnO, and SiO2. The surface science of metal oxides has been treated in an excellent monograph.[7] Unfortunately, the preparation of clean and well-ordered oxide surfaces on a bulk specimen still remains an experimental challenge.[1] The standard procedures for creating clean, well-ordered metal surfaces include polishing and etching, followed by ion sputtering to remove impurities and annealing to reestablish the crystal order. Such procedures, when applied to oxides, will, in many cases, lead to poor order or loss of stoichi-ometry due to preferential sputtering and/or thermally induced segregation.
As an alternative, in situ cleavage of bulk oxide specimens under UHV may be considered. However, materials such as SiO2, Al2O3, and TiO2 are difficult to cleave successfully in the sense that the cleavage plane will not coincide with an extended low-index plane, and that the surface may locally be very rough. Successfully cleaved surfaces can be used for one-shot experiments. To remove deposited metal, sputtering/annealing cycles are necessary.
Being large band gap materials the oxides are strongly insulating, adding to the difficulty and a severe drawback because when working with oxide surfaces most experimental techniques involve electron transfer, leading to charging effects. The charging problem has spurred the development of alternative schemes, where the oxide is made conductive by the introduction of point defects, or the oxide is grown in the form of an ultrathin film on a metallic substrate[1-4,8,9] that allows for electric transport through the film, e.g., in the form of a tunneling current.
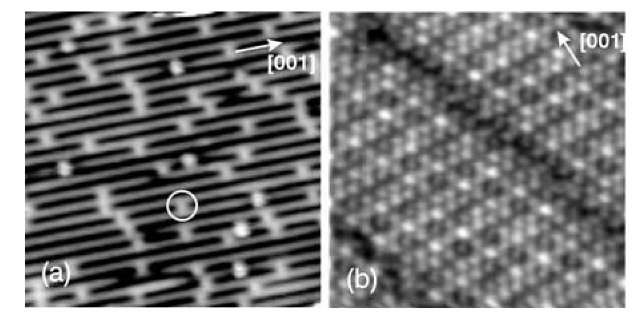
Examples of the two types of alternative schemes are shown in Fig. 2 in the form of scanning tunneling microscopy (STM) images of a TiO2 bulk crystal surface and an Al2O3 thin film surface. Titanium dioxide is used extensively, not only for heterogeneous catalysts, but in several other technologically important areas such as photocatalysis and sensor technology. Bulk TiO2 in its rutile form has a band gap of 3 eV, but thermal reduction under vacuum leads to oxygen deficiencies which create a shallow donor level in the gap, giving rise to a sufficiently increased conductivity for performing experiments such as STM on the surface.[7,8] The (110) surface of TiO2 in its rutile form shown in Fig. 2a is the most intensively studied TiO2 surface and it has become a prototype of a metal oxide.[10] Aluminum oxide (alumina), Al2O3, is extremely important in both ceramics and catalysis. A band gap of 89 eV makes Al2O3 an excellent insulator, and Al2O3 surfaces are not easily accessible for surface science experiments. Fortunately, thin alumina films can easily be prepared.[8,9] Oxidation at elevated temperatures, for example, of a (110) NiAl alloy single crystal surface results in the formation of an alumina film.[3,11] The ensuing film depicted in Fig. 2b has a thickness of only « 0.5 nm, less than the thickness of a unit cell of g-Al2O3 or a-Al2O3, but nevertheless sufficiently thick to have most of the properties of g-Al2O3.[3] And importantly, electron transport through the film is easily achieved, facilitating, e.g., STM investigations.
Fig. 1 High-resolution TEM micrograph of a ruthenium catalyst for ammonia synthesis. The almost circular dark regions are Ru metal clusters. The oxide is MgAl2O4.
Metal Deposition
The standard technique for metal deposition on extended oxide surfaces is vapor deposition, where a metal in close proximity to the oxide is heated to a temperature at which the metal has a suitable vapor pressure. Metal atoms will then impinge on the oxide surface at thermal energy. At room temperature (RT), the reevaporation probability for metal atoms is generally very low, i.e., the sticking probability is close to unity.
After landing, the metal adatoms may diffuse on the surface with a diffusion constant D determined by
for 2-D diffusion. Here v is a frequency factor of the order of 1013 Hz, a is the distance between neighboring adsorption sites, Ed is the energy barrier for diffusion, k is the Boltzmann constant, and T the absolute temperature. Diffusion can therefore be enhanced by an increase in temperature.
In a time interval t the diffusing adatoms will travel a root-mean-square (rms) distance I given by
For sufficient metal coverage the diffusion process leads to nucleation. If the diffusing metal atom attaches itself to a surface defect (such as a step edge), the nucleation is heterogeneous. If, on the other hand, diffusing adatoms meet and form stable nuclei, the nucleation is termed homogeneous. In general, only a few atoms need to agglomerate to form stable nuclei. The minimum number of other adatoms that a diffusing metal adatom must meet to form a stable nucleus is called the critical cluster (or island) size. Thus if a metal dimer is stable, the critical cluster size is one.
Fig. 2 STM images of oxides. (a) A clean TiO2 (110) surface (13 x 13 nm2). The bright lines are rows of Ti atoms. The bright dots imaged between the Ti rows (see circle) are vacancies in rows of oxygen atoms which are in bridge positions relative to underlying Ti atoms.
Continued metal evaporation does not lead to an ever-increasing density of clusters. The saturation density N is determined by parameters such as the rate R at which atoms impinge on the surface, the temperature T, the frequency factor v from the diffusion constant D (Eq. 1), the diffusion barrier Ed, and the critical cluster size i.[12] It is important to realize that for a given metal-oxide system, the saturation density of clusters can be controlled by changing the substrate temperature during nucleation and/ or the evaporation rate R.
When the saturation density of clusters has been reached, the clusters will grow by attachment of further adatoms. Although exceptions exist, the growth modes can conveniently be divided into three classes: Frank van der Merwe growth in which the metal grows layer-by-layer across the entire surface, Volmer-Weber growth in which metal grows as 3-D islands separated by bare oxide, and Stranski-Krastanow growth which starts as layer-by-layer growth, but with 3-D islands appearing after a few monolayers. Based on a model in which the cluster is treated as a liquid droplet on the support surface it is possible to have some preconception of the growth mode of a particular metal-oxide system. If gmet and gox denote the surface energies of the metal and the oxide, respectively, and gint the interface energy, the following rule can be established:
Expressions (3a) and (3b) apply to the thermodynamical equilibrium. It is important to note that film growth is a kinetic process which does not take place at thermody-namic equilibrium (growth is incompatible with equilibrium!). The expressions are therefore applicable only to growth conditions which are close to equilibrium. As the free energies of the mid-to-late transition metals and the noble metals are frequently larger than the free energies of the supporting oxides, 3-D growth rather than layer-by-layer growth is to be expected for such systems. This is, indeed, confirmed by experiments.[1] In the following, 3-D cluster growth is assumed.
Cluster Morphology
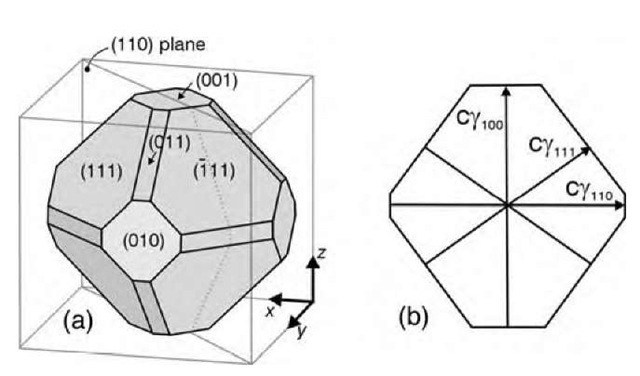
The thermodynamic equilibrium shape of a free metal cluster can be found from the Wulff theorem[14] if the surface free energies of the low-index surfaces are known. The basis of the theorem is that for a given volume, the equilibrium shape must be determined by minimizing the total surface free energy. The ensuing theorem states that the ratio between the real-space distance di from the cluster center to the facet plane i and the surface energy g of this facet is a constant:
Fig. 3 a shows a free cluster with a shape determined by the relative values of the surface energies g100, gno, and g111. A cross section of the cluster along the (110) plane is shown in Fig. 3b. The relative area of the different facets clearly depends on the relative values of g100, g110, and gm.
If the metal cluster is formed on an oxide, a metal-oxide interface is created. The free energy of the interface is given by the Dupre equation:
Fig. 3 a shows a free cluster with a shape determined by the relative values of the surface energies g100, gno, and g111. A cross section of the cluster along the (110) plane is shown in Fig. 3b. The relative area of the different facets clearly depends on the relative values of g100, g110, and gm.
If the metal cluster is formed on an oxide, a metal-oxide interface is created. The free energy of the interface is given by the Dupre equation:
where gint is the interface energy, gmet and gox the surface free energy of the contact facet of the cluster and the substrate, respectively, and Wadh the adhesion energy, i.e., the work per unit area needed to separate the cluster and the substrate.
The equilibrium shape of a supported cluster can now be found using a modified Wulff construction scheme,[15] by replacing the free energy of the surface in contact with the substrate with an effective surface energy g*, which is the difference between the interface energy and the surface energy of the oxide:
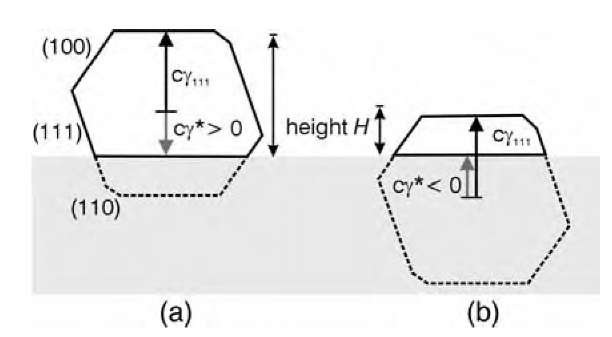
The height H of the supported cluster, relative to that of a free cluster, thus depends on g*. For a cluster residing on a {111} facet (Fig. 4), the cluster will adopt its free-space form on the oxide if g*=gm. For smaller values of g* the cluster gets truncated [Fig. 4a], and for negative values of g*, the height of the supported cluster becomes less than half the height of the free cluster, as illustrated in Fig. 4b. An experimental value of the g* can be derived from the observed, detailed morphology of the clusters in terms of the cluster surface energies.[16] From Eqs. 5 and 6
Fig. 3 The Wulff construction. (a) Cluster displaying three types of facets. (b) Cross section of the cluster along the (110) plane.
Fig. 4 Cross section of a cluster supported on a {111} facet for a positive (a) and a negative (b) value of the effective surface energy g*. H denotes the height of the cluster.
Again, gmet denotes the surface free energy of the metal cluster facet in contact with the oxide. The work of adhesion can therefore be derived from experiments, if the surface energies are known.
The Wulff construction is basically a continuum description which does not consider the discrete nature (finite number of atoms) of the clusters, and neglects the formation energy of corner and edge atoms, which play an important role in small clusters. Nevertheless, the theorem has been extremely successful in predicting or rationalizing shapes of metal clusters on oxides.
TWO CASE STUDIES
A large amount of metal/oxide combinations have been studied and an impressive amount of knowledge has been gained. In the following, focus will be on two different metal/oxide systems which have attracted special attention. The two systems will serve to illustrate fundamental properties, results, considerations, and problems related to research on metal clusters on oxides in general.
Palladium on Al2O3
Palladium on alumina is an important catalyst for partial or complete oxidation in many catalytic processes. The system has therefore been studied extensively, both in the form of industrial catalysts and as a model system. Most laboratory studies of Pd on alumina have been carried out on Al2O3 thin films because of the insulating nature of bulk Al2O3. Fig. 5a is an STM image showing the result of Pd evaporation onto a thin Al2O3 layer formed by oxidation of NiAl(110) (cf. Fig. 2b). As expected from surface free energy considerations, Pd grows in the form of 3-D clusters (Volmer-Weber growth). For room temperature, low-coverage deposition of Pd on well-ordered films, the spatial distribution of the clusters is heavily influenced by the intrinsic defect structure of the film with a tendency for the clusters to nucleate at step edges and domain boundaries.
From Fig. 5a it is evident that a large fraction of the clusters are hexagonal in shape. On films with large domains up to 50% of the clusters are hexagonal in shape,[16] reflecting the fact that these clusters are crystalline. The remaining clusters have not necessarily adopted their thermodynamical equilibrium form because of incorporated defects, insufficient temperature, etc. Because of the finite size of the STM tip the clusters are imaged as a convolution of the cluster and the tip shapes. This leads to a certain rounding of edges and corners as illustrated in Fig. 5b which displays height contours of a single Pd cluster.[17] Based on atomic resolution STM,[16] the facets of the cluster can be identified: The top facet is of {111} orientation while the sides are {111} and {100} facets. For comparison, Fig. 5c shows a ball model of a cluster. As discussed earlier, the general shape of the cluster is governed by the surface and interface energies (modified Wulff construction). The clusters do not display any {110} facet, either because g110 is too large compared to g100 and gm (cf. Fig. 3b), or because the cluster is too small to develop a genuine {110} facet. Fig. 5d shows the height contours of a Cu cluster formed under similar conditions.[17] In this case the shape of the top facet approaches a regular hexagon, illustrating how the surface energies influence the cluster morphology.
Fig. 5 (a) STM image of nanoclusters on Al203/NiAl(110). Note the characteristic hexagonal shape of many clusters. (50 x 50 nm2). (b) Height contours (thin lines) of a single Pd cluster. The thick line indicates the idealized, regular form. (15 x 15 nm2). (c) Ball model of a cluster displaying {100} and {111} facets. (d) Height contours (thin lines) of a Cu cluster, closely resembling a regular hexagon (thick line), (9 x 9 nm2). [(b) and (d) are reprinted from Ref. [17],
The principal objective for dispersing the active metal in a catalyst in the form of nanosized clusters is to increase the surface area. There are, however, several reasons why the dispersion, i.e., the formation of clusters, may influence the reactivity and make it deviate from that of corresponding, extended surfaces. First of all, the number of edge and corner sites will obviously increase dramatically. If such sites are active (see ”Gold on TiO2” section), the dispersion will promote an increase in activity which is easily explained. Secondly, exposure to gases may have different effects on extended surfaces and clusters dispersed on oxides. As an example, exposure of an extended Pd(111) surface to O2 is known to result in the formation of a (2 x 2) structure, i.e., a structure with a unit cell four times bigger than the substrate unit cell. Exposure of a clean Pd nanocrystal [Fig. 6a] to O2 has the same effect in the central portion of the top {111} facet, but an entirely new structure develops along the facet edge, as shown in Fig. 6b.[18] It is beyond the scope of this article to discuss this phenomenon in detail; suffice it to state that the adsorption of gases on nanoclusters may cause reconstructions along facet edges, thereby creating new, possibly catalytically active sites. For very small clusters, the presence of gas may therefore influence the structure of the entire cluster.
Fig. 6 Two atomically resolved STM images (2.5 x 2.5 nm2) of a Pd cluster before (a) and after (b) exposure to O2. The (2 x 2) oxygen adlayer in (b) is not imaged by STM, but a severe reordering of the facet edge has taken place.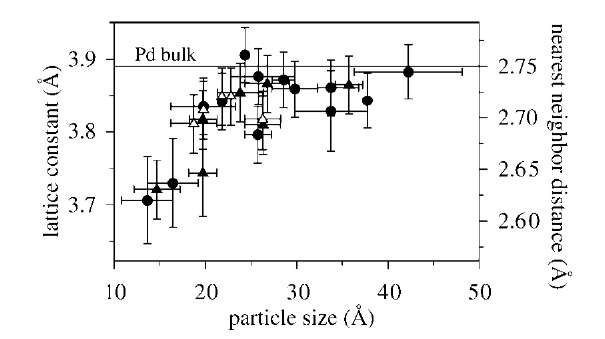
Fig. 7 Atomic spacing in Pd clusters on g-Al2O3/NiAl(110) as a function of cluster size. Horizontal bars illustrate the difference in length and width of the clusters whereas the vertical bars are estimated errors.
The previous example is related to adsorption under static conditions, i.e., at a fixed temperature and in an unchanging atmosphere of (low pressure) oxygen. What happens to the cluster morphology when the cluster is exposed to gases at a higher pressure and temperature, i.e., under industrial catalytic conditions? An answer was recently found based on an in situ investigation.1-19-1 Model catalysts of Cu nanoparticles on ZnO (equivalent to the industrial methanol synthesis catalyst) were imaged with TEM in a high-pressure cell. The Cu nanocrystals were found to undergo dynamic, reversible shape changes in response to changes in the gaseous environment. The changes were found to be caused by adsorbate-induced changes in the surface energies and by changes in the interface energy. To describe and understand the catalytic properties of nanoscale clusters, dynamic effects on the nanocrystal morphology must therefore be included.
Dispersion may lead to effects of an even more subtle origin. Consider, for example, the sensitivity to changes in the lattice parameter. Because of the increased importance of the surface stress for smaller clusters, the lattice parameter is expected to decrease for decreasing cluster size.[20] Fig. 7 shows an experimental verification obtained for Pd on Al2O3 on the basis of TEM mea-surements.[21] The smallest clusters (size «1.5 nm) show a 4-5% reduction in lattice parameter. A smaller lattice parameter results in a broadening of the d-band. Compare to the effect of bringing atoms together to form a solid: The atomic levels broaden and become energy bands. The closer the atoms the stronger the broadening. For transition metals with d-bands that are more than half-filled, such as Pd, the broadening results in a downward shift of the d-band center to maintain the degree of filling. According to the d-band model, a shift in the d-band center relative to the Fermi energy will be correlated with a change in binding energy.[22] Hence a change in the lattice parameter will influence the reactive properties of the cluster. An indisputable experimental verification of this effect on clusters is still lacking, but changes in the reactivity of a metallic surface induced by a change in lattice parameter has been reported for a (0001)-oriented Ru sample in an area where an edge dislocation intersects the surface.[23] On one side of the dislocation the lattice is compressed, and on the other side it is stretched. When analyzing the results of NO dissociation on the surface with STM, a much larger concentration of N atoms appeared at the stretched than at the compressed and the defect-free surface, signaling an enhanced reactivity of the stretched surface in accordance with the d-band model.[22]
For tiny (few-atom) clusters, the electronic structure cannot be properly described in a band structure model. Instead the energy levels may be described in a picture based on molecular orbitals. The energy levels, and hence the reactivity, will therefore be very sensitive to the detailed size and shape of the cluster.
In this section a number of concepts which are central in the description of catalytically active metal clusters have been described, whereas the role of the substrate has been neglected, apart from its part in providing a passive, high surface-area matrix (structural promoter) for the dispersed metal clusters. Increasing evidence has been found that the oxide in many cases plays a much more active and decisive role than hitherto expected. References to some aspects of metal-oxide interaction will be made in the next section.
Gold on TiO2
Gold is generally accepted to be the noblest of all the metals because of its electronic structure with a filled 5d band.[24] Only little attention has therefore been paid to gold as a potential catalyst. Nevertheless, when gold is highly dispersed on select oxide surfaces in the form of nanosized clusters, its chemistry changes dramatically and surprisingly a high activity and/or selectivity is exhibited in catalytic processes such as the low-temperature oxidation of CO.[25] The observation that the chemical properties of gold depend on the support and in particular on the size of the Au clusters has spurred an intense search for the underlying physical origin.
When Au is evaporated onto a TiO2 surface (cleaned by Ar ion sputtering and vacuum annealed to «1000 K), the Au atoms form clusters that nucleate at defects. Apart from major defects such as steps, the main nucleation sites are the bridging-oxygen vacancies shown in Fig. 2a.[26] A single oxygen vacancy will bind up to three Au atoms and for larger Au clusters, the Au/ TiO2 interface will contain a high density of oxygen vacancies.
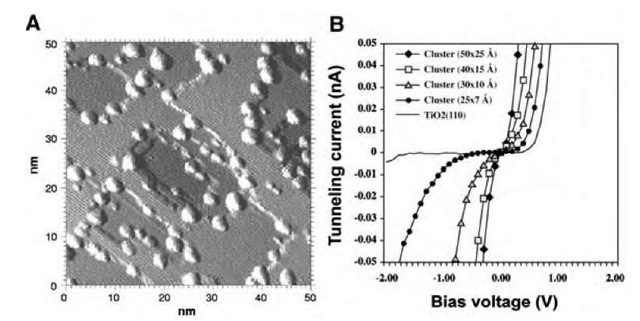
Fig. 8A shows an STM image of a 50 x 50-nm area of a TiO2 (110) surface with a large number of Au clusters (bright protrusions).[27] As already mentioned the average cluster size can be varied by adjusting the evaporation flux, temperature, and total dose. By measuring the turnover frequency (TOF), i.e., the number of reactions taking place per Au atom per second, for CO oxidation as a function of the Au cluster size, a clear correlation between the TOF and the cluster diameter was revealed, showing a distinct maximum for a Au cluster diameter of « 3 nm.[27]
In the search for the origin of this effect, the electronic properties of the Au clusters were investigated with scanning tunneling spectroscopy (STS).[27] In this technique, the STM tip is positioned on top of a particular cluster, and the tunnel current I is recorded as a function of the tunnel voltage V. The resulting I-V curves reflect the electronic properties of the clusters and can be correlated with cluster size and geometry, as shown in Fig. 8B. A perfectly metallic cluster on a conducting substrate should display a simple ohmic behavior, i.e., a straight line as I-V curve. From Fig. 8B it is evident that the smaller the cluster the stronger the deviation from simple ohmic behavior is observed. When the dimension of the cluster is decreased, a plateau of zero tunnel current develops around zero bias voltage corresponding to the appearance of a band gap between the valence and the conduction band. For comparison, an STS curve for the TiO2 substrate, having a wider band gap than the Au clusters, is also included in Fig. 8B. These measurements provide a fine illustration of how quantum size effects become important when the dimensions of metal clusters are reduced, but do not by themselves explain the peak in activity for a given cluster size.
Fig. 8 (A) Constant-current STM image of Au/Ti02(110). The total amount of Au corresponds to 0.25 monolayer. (B) Scanning tunneling spectroscopy data acquired for Au clusters of varying sizes on the Ti02(110) surface. For reference the STS data of the TiO2 substrate are also shown.
To elucidate the mechanism behind the observed activity, the possible role of the substrate must also be considered. It is well known that the support may take part in reactions, for example via spillover effects where reactants may be formed at the metal clusters or the oxide and migrate from one to the other. The active role of an oxide support during CO oxidation over supported Au catalysts has been investigated theoretically and experimentally with conflicting results (see Ref. [28] and references therein). To illustrate the complexity of the problem, reference is made to a case study: a theory study based on density functional theory (DFT) calculations.[29] MgO rather than TiO2 was chosen as a substrate because of its structural simplicity and appearance in the experimental literature (Au clusters on MgO have also been shown to be reactive to CO oxidation at RT and even below.)[30] The higher reactivity of supported nanometer-sized Au clusters was found to originate from two effects: 1) the oxide acts as a structural promoter, leading to the formation of small Au clusters with low-coordinated Au edge sites that readily bind CO; 2) the proximity of the oxide stabilizes a peroxo-like reaction intermediate COO2.
It remains to be seen how general such mechanisms are, but the example serves to illustrate how the catalytic process may rely on an intricate interplay between the metal cluster and the oxide, and how challenging it is to sort out the relative importance of different effects.
CONCLUSION
In this article, emphasis has been on the synthesis and characterization of metal clusters on relatively flat, extended oxides which mimic real, metal-based catalysts sufficiently well to be exploited as model systems. Based on two case stories, Pd/Al2O3 and Au/TiO2, a number of issues relevant for such systems have been introduced and discussed. Many other fascinating applications of metal clusters on oxides may be developed in the near future. Examples can already be found in the literature, such as the fabrication of single-electron transistors based on contacting Au nanoclusters on SiO2 with carbon nano-tubes.[31] While each example is of interest in its own area, the principal importance of metal clusters on oxides is unquestionably still in the field of catalysis.

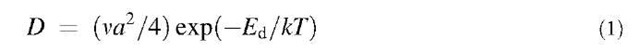



![(a) STM image of nanoclusters on Al203/NiAl(110). Note the characteristic hexagonal shape of many clusters. (50 x 50 nm2). (b) Height contours (thin lines) of a single Pd cluster. The thick line indicates the idealized, regular form. (15 x 15 nm2). (c) Ball model of a cluster displaying {100} and {111} facets. (d) Height contours (thin lines) of a Cu cluster, closely resembling a regular hexagon (thick line), (9 x 9 nm2). [(b) and (d) are reprinted from Ref. [17], (a) STM image of nanoclusters on Al203/NiAl(110). Note the characteristic hexagonal shape of many clusters. (50 x 50 nm2). (b) Height contours (thin lines) of a single Pd cluster. The thick line indicates the idealized, regular form. (15 x 15 nm2). (c) Ball model of a cluster displaying {100} and {111} facets. (d) Height contours (thin lines) of a Cu cluster, closely resembling a regular hexagon (thick line), (9 x 9 nm2). [(b) and (d) are reprinted from Ref. [17],](http://what-when-how.com/wp-content/uploads/2011/03/tmp25368_thumb_thumb.jpg)