Introduction
Law enforcement officers and the general public associate fingerprints, more than any other category of physical evidence, with criminal investigations and apprehension of criminals. Fingerprints definitely prove the presence at a crime scene of the individual who made the impressions, and they establish that said individual once had contact with a given item now constituting evidence.
Each ridge of the fingers and palms, and the soles of the feet bears a row of sweat pores which constantly exude perspiration (Fig. 1). In addition, the ridges of the fingers and the palms are in intermittent contact with other parts of the body, such as hair and the face, and with various objects, which may leave a film of grease or moisture on the ridges. When an object is touched, an outline of the ridges of the fingers or palm is deposited. This outline is called latent impressions, or latent fingerprints.
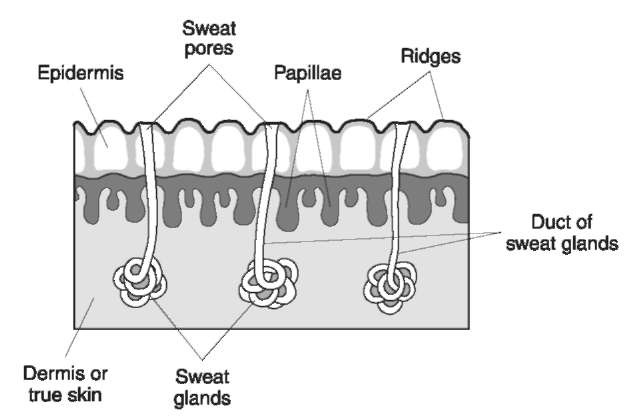
Figure 1 Cross section of friction skin.
Latent prints are, perhaps, the most prominent example of Locard’s exchange principle: ‘Every contact leaves traces’; hence, the great importance of visualizing traces and transferring them onto useful and valuable evidence.
The detection and imaging of latent fingerprints is unique in forensic science. The objective is not to detect and quantify specific chemical substances per se, but rather to map the distribution of these chemicals on various surfaces. Indeed, one of the most exciting and dynamic areas of research in forensic science today is the application of physicochemical techniques to the visualization of latent fingerprints. Changes are occurring very rapidly as researchers uncover a variety of processes applicable to the visualization of latent prints. For many years progress in this field was minimal, as fingerprint specialists traditionally relied on three chemical techniques -iodine, ninhydrin and silver nitrate – to reveal the presence of fingerprints. Many more techniques have been developed over the last two decades, and special attention has been given to techniques based on fluorescence, or fluorogenic processes. In designing improved fingerprint reagents, it is essential to have a sound knowledge of the chemical composition of both fingerprint deposits and of the surfaces on which they are found. Much work in this aspect was conducted by the Police Scientific Development Branch (UK) in the 1970s.
To achieve successful results, it is not necessary for a fingerprint technician to know the exact chemical composition of a latent print residue or the chemical processes involved. In the majority of instances, they must see the surface to be examined, and then decide on the proper sequence in applying chemical techniques.
Not all attempts to recover latent fingerprints are successful, even with proper handling and application of the appropriate fingerprint techniques. Failure to recover latent fingerprints does not, in itself, necessarily mean that there were no prints on the item or that they were wiped off. The inability to recover latent prints can be due to numerous factors. It is a function of perspiration of the individual leaving the latent mark, surface type, environmental conditions and the age of the print.
Chemical Composition of Fingerprint Deposits
A latent print is a complex mixture of natural secretions and environmental contaminants. Eccrinic, apocrinic and sebaceous glands are responsible for natural secretions of the skin. Of these, only eccrinic glands are found in ridged skin, where they are abundant. Studies have shown that there are 550-950 sweat pores per square centimeter in finger ridges, and less (400) in the palms and soles.
The apocrine glands are found in the armpits, chest, abdominal and genital areas, whereas sebaceous glands are found on the forehead, around the nose, on the back and genital areas.
As a result, eccrinic gland secretions are present to some degree in every latent fingerprint; contamination by sebaceous secretions (sebum) is also very common from people touching their faces.
Approximately 99.0-99.5% of perspiration is water. The remaining residue consists of small amounts of organic and inorganic materials. The salts predominant in perspiration are sodium and potassium chlorides, with the organic fraction containing mainly amino acids, urea and lactic acid. Free fatty acids, triglycerides and wax esters prevail in sebaceous secretions (Tables 1-3).
Table 1 The main constituents of the secretion of the sweat glands (excluding water)
| Gland type | Inorganic | Organic |
| constituents | constituents | |
| Eccrine | Chlorides | Amino acids |
| Sodium | Urea | |
| Potassium | Lactic acid | |
| Ammonia | Sugars | |
| Sulfates | Creatinine | |
| Phosphates | Choline Uric acid | |
| Apocrine | Iron | Proteins Carbohydrates Cholesterol |
| Sebaceous | Fatty acids Glycerides Hydrocarbons Alcohols |
Table 2 Levels of amino acids, chlorides and urea in fingerprint deposits
| Compound | Levels in fingerprints (\igcm 2) | Levels in 120 types of paper (\igcm 2) |
| Amino acids (as serine) | Range 0.120-0.720 | 0.004-1.000 |
| Mean = 0.250 | ||
| Chlorides | Range 0.270-1.500 | 0.500-6.000 |
| Mean = 0.670 | ||
| Urea | Range 0.090-0.720 | No detectable levels in the papers tested |
| Mean = 0.250 | in this study. Some surface-coated | |
| papers do have high levels of urea. |
Table 3 Main lipid classes in sebum
| Component | % |
| Free fatty acids | 30 |
| Triglycerides | 27 |
| Diglycerides | 4 |
| Monoglycerides | 2 |
| Wax esters | 22 |
| Squalene | 10 |
| Cholesterol | 2 |
| Cholesterol esters | 2 |
| Hydrocarbons | 1 |
In addition to natural secretions, latent fingerprints may often be contaminated by other materials present in the environment and picked up on the skin. It is usually impossible to determine the major constituents from simple visual examination, hence, apart from when the marks are obviously made in a contaminant such as blood, ink, dust or grease, the latent prints are considered to contain mainly sweat.
The possibility of visualizing a latent fingerprint depends on the constituents of the original fingerprint (the individual person), the nature of the surface, the time elapsed since deposition and the storage conditions. Factors such as temperature, humidity and exposure to light and dust have a strong adverse effect on the durability of latent prints.
Latent Fingerprint Techniques: Development of Latent Fingerprints
A latent fingerprint technique is any method, process or procedure used to visualize or enhance latent fingerprint impressions for preservation and identification.
Despite the wide range of surfaces and detection methods described in the literature, even the most advanced laboratories do not usually employ more than ten different methods that are carried out in various combinations and modifications. These are: visual examination, dusting with powders, cyanoa-crylate fuming, vacuum-metal deposition and, occasionally, iodine, for smooth, nonabsorbent surfaces; and visual examination, diazafluorenone (DFO), nin-hydrin, physical developer and, occasionally, iodine for porous surfaces, such as paper and cardboard. Additional methods used less frequently are silver nitrate (for rough surfaces such as wood), small particle reagent (SPR) (for wet, smooth or dusty surfaces), and ‘secondary’ methods such as contrast enhancement by fluorescent powders, or intensely colored substances such as gentian violet. Weak prints in blood can be enhanced by chemical reagents, for example amido black, luminol or tetramethyl benzi-dine. Since the person conducting the test will normally seek the optimal development method for the surface to be examined, the range of tests is divided according to surfaces and the best test for each.
Only rarely will just one test detect all the latent prints on a surface, though sometimes there is no practical way to employ multiple methods (cost-effectiveness considerations or pressure of time). At present, at least in the investigation of serious crimes, forensic science laboratories will employ a battery of detection methods on each surface. The methods are used in sequence and after each stage, should prints develop, the results are photographed and the process continued.
Obviously, the process commences with the nondestructive methods followed by methods that are only partly destructive, and concludes with techniques that leave permanent traces. Another consideration for the sequence of methods chosen is that a prior method should not interfere with a later method.
General, nondestructive techniques: visual examination by optical means
Optical detection has the advantage of not destroying surfaces and latent fingerprint deposits. As a result, these techniques do not preclude the later application of other fingerprint techniques. Observation under white light may reveal latent fingerprints that can be photographed without any further treatment. More complex optical methods may disclose prints that are otherwise invisible.
White light detection White light, particularly in strong illumination, is one of the most useful tools in fingerprint visualization. Strong white light illumination is used in nearly every case in which fingerprint evidence is sought. Proper alignment between the light source and field of vision is normally determined by trial and error. A print can be invisible with the light in one position, but may be most apparent with the same light in another position.
Episcopic coaxial illumination Highly reflective surfaces, such as glass, polished metals and certain plastics, pose a problem in fingerprint detection under white light due to reflection. In such cases, latent prints can often be detected using episcopic coaxial illumination. This is a special illumination technique which uses a semitransparent mirror to observe the reflection of light perpendicular to the surface (Fig. 2). The light is diffused by the fingerprint deposit, but specularly reflected by the surface. The print is therefore visible as dark ridges against a light background. This technique also gives excellent results after cyano-acrylate treatment.
Ultraviolet illumination Ultraviolet illumination as a nondestructive detection method for latent fingerprints is far less common, since natural fingerprint deposits do not showany particular response in this domain. Certain environmental contaminants, however, such as greases or food ingredients do fluoresce under long-wave UV illumination.
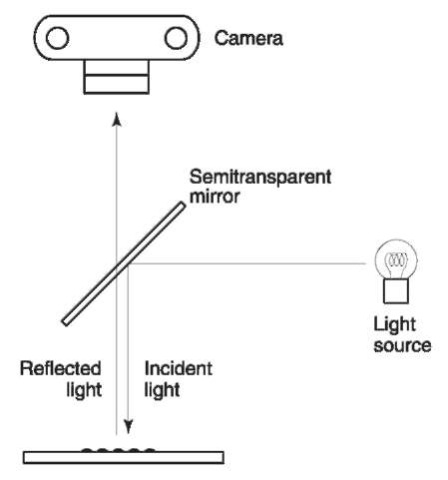
Figure 2 A diagram for fingerprint detection by episcopic coaxial illumination.
Due to the fact that today UV light sources are inexpensive, easily operated and readily available, their use (long wavelength, 354 nm) in many laboratories is a common, nondestructive procedure after examination in white light.
Detection systems that involve short-wave UV illumination (<300nm) have been described in the literature. Detection is based on either reflection of the fingerprint deposit, which differs from that of the background, or on the induced short-wave UV fluorescence of certain sweat components. The detection system involves a strong source of short-wave UV light (frequency quadrupled Nd:YAG lasers or mercury lamps) and UV-sensitive CCD camera equipped with quartz lenses. Although there are some commercially available systems that use short-wave UV illumination for fingerprint detection, these are still considered experimental. In addition, they are not totally nondestructive, since prolonged illumination by short-wave UV light may irreversibly alter DNA from the exhibits. For the same reason, special safety precautions must be employed for short-wave UV work, such as the wearing of safety goggles and skin protection gear.
Lasers and alternate light sources A most important development in the field of latent fingerprint detection was the discovery in 1976 that latent fingerprints on various surfaces could be revealed by their inherent luminescence when illuminated with an argon-ion laser.
It was later found that in actual casework only a small fraction of untreated prints could be visualized by this technique. Nevertheless, if an argon-ion laser is used, it should always precede more destructive methods. The detection is based on the presence of luminescent compounds, such as riboflavin, in the sweat components, but it is more likely to obtain good results when the fingers are contaminated with foreign luminescent substances. An outstanding example of the value of this nondestructive technique was the detection, by the FBI Laboratory in 1984, of the 42-year-old thumbprint of the war criminal, Valerian Trif a, on a postcard sent to Heinrich Himmler in 1942. The procedure used to detect latent prints with the laser is relatively simple, and evidence is not altered. Latent prints have been detected by their inherent luminescence on a wide variety of surfaces, including metals (e.g. firearms), paper, adhesive tapes, plastic and polystyrene foam. Positive results have been reported even on human skin.
The light beam emitted from the argon-ion laser (green and blue-green in color, the main two lines, 488 and 514.5 nm) is dispersed by a fiberoptic cable to cover an area of approximately 10 cm in diameter.
The illuminated area is viewed through a long-wavelength-pass filter that transmits the fingerprint fluorescence but blocks the reflected laser light. Observed fingerprints are photographed through the same filter (Fig. 3).
Alternate light sources for fingerprint detection began to appear in the mid 1980s. They were smaller, lighter, less expensive and more diverse than the lasers. In 1983, in Australia, a modified xenon arc lamp was developed. At about the same time, the ‘Quaser’ light source was developed by the Scientific Research and Development Branch of the UK Home Office, and the ‘Lumaprint’ lamp was designed at the National Research Council of Canada. In many forensic laboratories alternate light sources (also referred to as ‘forensic lights’) have become the main nondestructive tool for the search of latent fingerprints.
These devices are, basically, powerful sources of white light equipped with an appropriate set of filters, which transfer only the desired fraction of light that hits the material under examination and excites fingerprint or background fluorescence. Although their performance in fingerprint visualization is generally comparable with that of lasers, it was reported by groups in Israel and Canada that, in actual casework, latent prints were encountered that could not be visualized by the alternative light sources whereas the argon-ion laser or the copper-vapor laser did detect the prints. However, the main contribution of lasers and their substitutes to fingerprint visualization is not in the detection of inherent fingerprint luminescence, but in the excitation of luminescence after the latent prints have been chemically treated by fluorescent or fluorogenic reagents.
Visualization techniques for smooth, nonporous surfaces
Surfaces such as glass, paint, plastic and metals are included in this category.

Figure 3 A diagram for fingerprint search by argon-ion laser.
Powder dusting The simplest and most commonly used procedure for latent fingerprint development is powder dusting. Visualization is based on the physical adherence of fingerprint powders to the moisture and oily components of skin ridge deposits, but the exact mechanism is not fully understood. Application of powder to latent prints by brushing is a simple technique and yields instantly apparent prints. Only minimal training is required to obtain satisfactory results. Powder dusting, however, is considered an insensitive technique and only relatively fresh latent prints are normally developed. Hundreds of fingerprint powder formulae have been developed over the years. Aluminum powder is composed of microscopic aluminum flakes coated with stearic acid. Fluorescent powders luminesce under various wavelength illumination and may be used on reflective surfaces. Magnetic powders are composed of iron flakes, mixed with copper or aluminum flakes; they are applied by a magnetic wand which allegedly avoids brushing and the destruction of fragile prints. Choice of the appropriate powder and application method is often made according to experience and personal preference. Fingerprint brushes are made of hairs, feathers or fiberglass. Fiberglass brushes last longer than the other two types, and have gained wide acceptance in recent years.
Cyanoacrylate fuming In 1977, the National Police Agency of Japan made an amazing discovery that had an immense impact on the art of latent fingerprint detection. They noticed that fumes of the high-strength quick glue, ‘Superglue’, developed latent fingerprints by selectively polymerizing on the ridges in the form of a hard, white crust. This technique was quickly adopted by fingerprint practitioners the world over and also received much attention from researchers who optimized its performance and extended its range of application.
‘Superglue’ is composed basically of a cyanoacrylate ester, normally methyl or ethyl cyanoacrylate. These are colorless liquids with relatively high vapor pressure. Cyanoacrylate fumes polymerize upon reaction with latent fingerprint deposits. The polymerization is thought to be catalyzed by the water and possibly other perspiration constituents (for mechanism, see Fig. 4).
The use of cyanoacrylate vapor has greatly enhanced the possibility of developing latent prints on most nonporous surfaces, especially plastic bags and other pliable plastics, but also on styrofoam, carbon paper, aluminum foil, finished and unfinished wood, rubber, metals and even smooth rocks. The contrast of developed fingerprints may be enhanced by the application of dyes and powders.
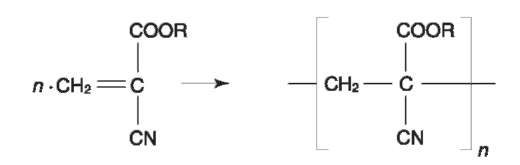
Figure 4 The cyanoacrylate process.
Equipment required for cyanoacrylate fuming includes a fuming tank, cabinet or other suitable container equipped with a proper ventilation system. The samples to be treated are suspended in the tank, and a fewdrops of liquid cyanoacrylate are dripped into a container at the bottom. A container of water is also placed in the tank to insure sufficient humidity for the development process (prints of poor contrast are obtained if the humidity is too low). Variations on this procedure involve applying heat or chemicals to accelerate the vaporization; varying exposure times for optimal results; controlling the generation of fumes under lowpressure (‘vacuum cyanoacrylate’); using portable field devices to fume large objects; staining techniques for improved contrast; the use of the polymer, polycyanoacrylate, as the source of the fumes; incorporation of cyanoacrylate into a gel matrix (Superglue ‘pouch’); using various alkyl groups in the ester (ethyl ester is the most common); and removal of excess polycyanoacrylate from prints overexposed to Superglue.
Vacuum metal deposition (VMD) Vacuum metal deposition (VMD) utilizes vacuum coating technology for the evaporation of metals and the deposition of thin metal films. It is based on a phenomenon that has been a great nuisance in industrial growing of thin layers of metals on various surfaces, where fingerprint contamination hinders the deposition of the metallic film following metal evaporation under vacuum. The potential of this method for fingerprint detection was first reported in 1968, and thoroughly investigated a fewyears later but practical work started in 1976.
VMD is considered an extremely sensitive and useful technique for fingerprint detection on a variety of smooth, nonporous surfaces, particularly plastic packaging materials such as polythene, leather, photographic negatives and prints, plastic moldings and glass.
Gold is evaporated under vacuum to form a very thin, invisible layer on the surface under examination. A second layer, this time, zinc, is deposited in the same manner. The zinc preferentially coats the regions between and around the ridges, where the gold is more exposed. Thus, the ridges appear transparent whereas the furrows and the background are dark (since they are plated with a zinc layer). Interestingly, the original second metal that gave excellent results was cadmium but, due to its toxicity, it was replaced by zinc.
VMD can sometimes reveal fingerprint detail where other techniques have failed. Good results can also be obtained after cyanoacrylate development.
One of the advantages of VMD is extreme sensitivity, even in cases of old fingerprints and prints which have been exposed to water. Disadvantages are the initial cost of high vacuum equipment, the need for expert operators, and the fact that it is a most time-consuming process.
Iodine fuming Iodine fuming is one of the oldest methods used for the chemical development of latent fingerprints. Its use was mentioned as early as 1892. It can develop prints quickly and neatly on porous and nonporous surfaces.
Iodine vapors are absorbed by fingerprint residue to form a brown image which fades rapidly. For many years, it was assumed that iodine adds to the double bonds of the unsaturated fatty acids present in perspiration, but there is convincing evidence that this is a physical, rather than a chemical, reaction, and that iodine fumes are also absorbed by other sweat components, such as saturated fatty acids and even water. The technique is simple but not very sensitive. It reveals latent prints not more than a few days old. The developed prints must be photographed immediately before they fade. Alternatively, they can be fixed by chemical means such as benzoflavone or tetrabase. Although the iodine fuming method has been ignored somewhat due to more modern, faster techniques such as lasers and cyanoacrylate fuming, it remains an important method due to its simplicity, speed and lowcost. Disadvantages are lowsensitivity to old prints and the toxic and corrosive nature of the fumes.
Iodine fumes are obtained by slightly warming iodine crystals. Control of the fumes is achieved by using the crystals in an iodine gun or fuming cabinet. Iodine blowing guns are made of a glass or hard plastic tube. A drying agent such as calcium chloride is used to dry the vapors. The iodine crystals sublime by the heat of the breath, which is augmented by the warmth of the hand which is cupped around the tube containing the crystals. The vapor is blown onto the specimen. Fuming cabinets are normally made of glass. The fumes are generated by placing a small heating device under an evaporating dish containing the iodine crystals. The specimens are then suspended above the dish.
Iodine solution method modification of the fuming technique, that uses iodine in solution, is used by law-enforcement agencies in the UK. A solution of iodine in cyclohexane containing 7,8-benzoflavone is used at crime scenes to search for latent fingerprints on surfaces such as wallpaper, emulsion-painted walls and aged glass-painted surfaces. The reagent is applied by spraying or brushing, and the fingerprints showup as dark-blue ridges. This technique is particularly effective for revealing fresh marks.
Visualization techniques for porous surfaces
The main surfaces in this category are paper and cardboard. The search for latent prints must start, as for nonporous articles, with nondestructive visual examinations and continue with chemical techniques that can be augmented by appropriate light sources.
Ninhydrin Perhaps the most meaningful breakthrough in the chemical detection of latent fingerprints on paper occurred in 1954, when ninhydrin (Fig. 5) was proposed for the development of latent fingerprints. Since then ninhydrin has become the most consistently successful reagent for developing latent fingerprints on documents and other porous surfaces. Ninhydrin reacts with amino acids to produce a highly colored compound known as Ruhe-mann’s purple (named after Ruhemann, the scientist who discovered and interpreted this reaction in England, in 1910).
Amino acids on paper are relatively stable and do not migrate with age. Ninhydrin is, therefore, a most suitable reagent to reveal even old prints on paper. Its use is simple, but skill and training are required to obtain good results.
In 1955, a formulation for fingerprint development was proposed which consisted of acetone as a solvent and acetic acid to enhance sensitivity. Since then, numerous formulations and development conditions have been investigated by various researchers. Among the parameters studied were: solvents, concentrations, temperatures and heating times, pH, modes of application and humidity conditions. Currently, the most common formulation is a modification based on the nonpolar solvent 1,1,2-trifluorotrichloroethane (fluorisol, freon 113). The use of freon 113 virtually eliminates the problems of ink running and is also nonflammable. This formulation is known by the abbreviated process name NFN (nonflammable nin-hydrin). Due to its adverse effect on the ozone layer,freon 113 is nowbanned in many countries and alternative solvents are currently being studied.
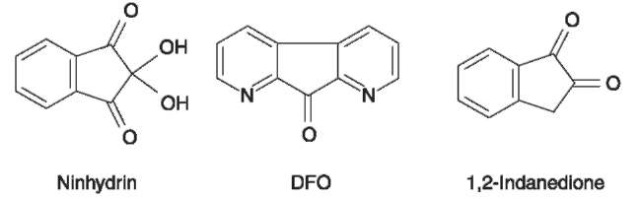
Figure 5 Formulae for ninhydrin; 1,8-diaza-9-fluorenone (DFO) and 1,2-indanedione.
Generally, items being examined are dipped into ninhydrin solution for a fewseconds, dried in air, then warmed in an oven at temperatures not exceeding 80°C and c. 65% relative humidity. Fingerprint marks appear as purple impressions. The reagent may also be applied by spraying or brushing. Weak ninhy-drin prints can be improved by converting them into fluorescent impressions by treatment with zinc chloride. This process, originally suggested in 1982, and further study suggested the use of cadmium instead of zinc. The structure of the fluorescent complex was clarified in 1987.
DFO (l,8-diaza-9-fluorenone) DFO is one of the most recent and significant developments in reagents for the chemical development of latent fingerprints. Its introduction into practical work has brought about a considerable increase in the number of latent fingerprints that can be revealed on paper items. Its specific reactivity was originally discovered by Grigg (University of Belfast) and Pounds (CRE, British Home Office), during a project on ninhydrin analogues. Among other compounds, the heterocyclic ketone, 1,8-diaza-9-fluorenone (DFO, Fig. 5) was studied and its solution was found to produce strong luminescent impressions of latent fingerprints on paper. A mechanistic explanation to the fluorogenic process was also suggested, based on a reaction between DFO and amino acids.
Some advantages of the DFO reagent are high sensitivity, relatively broad excitation and emission bands, adequate solubility in nonpolar solvents and the possibility of using ninhydrin and physical-developer after the application of DFO. In addition, there is no need for secondary treatment with zinc to excite the luminescence. To develop latent fingerprints, paper and cardboard items are dipped in a dilute solution of DFO in freon 113 and acetic acid. The articles are heated in the oven and illuminated by an argon-ion laser or one of the alternate light sources emitting in the blue, blue-green or green domain. Observation of the luminescent impressions is through a cut-off filter that transmits light above 520 nm. In some cases, the developed prints may also have a pale purple color, which is much weaker than that of ninhydrin-developed prints, but may indicate the presence of latent fingerprints on the examined article.
Physical developer (PD) Physical development is a photographic process based on the formation of gray silver deposits from an aqueous solution containing silver ions in the presence of ferrous/ferric redox couple and a detergent. A reviewof the early work on techniques using stabilized physical developer for fingerprint development found that metallic silver is deposited on the lipid material present in the fingerprint residue. The detergent prevents premature precipitation. It was later found that the process can also be used after treatment with DFO or ninhydrin. It can produce a significant number of extra prints, since PD reacts with different constituents of the fingerprint deposit. It is a very effective reagent for developing latent fingerprints on wet paper. Other articles that can be processed by PD are adhesive strips on envelopes, adhesive tapes, the emulsion side of photographs, and certain types of currency notes. PD reagent is delicate to prepare, since the solution is not very stable. Also, silver sometimes precipitates on the entire surface of the paper. Hence, PD should be applied at the end of any detection sequence on paper. Its successful application requires considerable experience. The working solution must be prepared carefully in clean glassware and only good quality distilled or deionized water should be used. The detergent contains N-dodecylamine acetate and synperonic NP8; silver nitrate is the source of silver ions, and the redox couple is a mixture of ferric nitrate and ferrous ammonium sulfate in dilute citric acid. The sample must be washed in distilled water to remove surface contamination before it can be treated with the reagent. Certain paper items require a prewash with maleic acid to reduce background discoloration. The development process occurs by soaking the articles in the reagent, rinsing with distilled water, and drying.
Modified PD Several modified PD procedures have been reported: the use of scanning electron microscopy for observation (to reduce background interference); conversion of the silver deposit to radioactive silver sulfide and recording the image by autoradiog-raphy; and particularly, Saunders’ and Cantu’s ‘mul-timetal deposition’ in which colloidal gold provides a nucleation site for the silver precipitation.
Silver nitrate One of the oldest methods for chemical development of latent fingerprints, silver nitrate treatment, has been in use since 1891. Silver ions react with the chlorides present in eccrine perspiration. The colorless silver chloride thus formed turns black rapidly on exposure to light. The procedure is simple but it may produce a high background reaction which obscures fingerprints. In light of more advanced chemical methods such as DFO, ninhydrin and physical developer, the silver nitrate technique is considered obsolete, at least for paper items, by many forensic science laboratories. It may still be of some value on rawwooden surfaces.
Small particle reagent (SPR) Small particle reagent, also known as ‘suspended particle reagent’ (SPR) consists of a suspension of fine molybdenum disulfide particles in detergent solution. The particles adhere to the fatty constituents of latent print residues to form a gray molybdenum disulfide deposit. The method was patented in 1979. Its great advantage is that it can develop latent prints on nonporous, dusty or wet surfaces, where the use of powders is excluded. It can be applied in a dish or by spraying. The latter technique is less sensitive and should be considered only when dish application is impossible. White SPR, based on fine zinc carbonate particles, can be used on dark surfaces, as well as fluorescent SPR.
Other methods Additional special techniques have been designed for fingerprint development on problematic surfaces. For example, the radioactive sulfur dioxide method was developed to visualize latent fingerprints on fabrics and adhesive tapes. Its application requires special facilities and trained staff, hence, it is used only in high-profile cases.
The National Police Agency of Japan uses ninhy-drin hemi-acetal, a nonpolar derivative of ninhydrin in petrol-ether solution, to develop latent prints on ‘thermal’ paper. The US Secret Service and the Metropolitan Police Forensic Science Laboratory (London) advocate subliming p-dimethylamino-cinnamaldehyde (p-DMAC) for the same purpose. A suspension of Sudan black can be used to develop sebaceous components of latent prints on surfaces contaminated with grease or foodstuffs. Many more variations are in routine use by fingerprint practitioners. Some latent fingerprint techniques mentioned in the professional literature, but rarely used, are listed below. Osmium tetroxide (which is extremely toxic) reacts with unsaturated fatty acids, to deposit, on the ridges, dark osmic acid impressions on both porous and nonporous surfaces. Ruthenium tetroxide reacts in a fashion similar to osmium tetroxide. It is less toxic than osmium tetroxide but is, nevertheless, an eye and respiratory tract irritant. p-Dimethyl-aminocinnamaldehyde (p-DMAC) reacts with urea present in latent print deposits on paper, to form red impressions. Due to the migration of urea from the deposits, it only reacts well with fresh prints. (p-DMAC is nowconsidered to be a much more efficient amino acid fluorogenic reagent in the vapor phase.)
Heat has been mentioned by several experts as a latent fingerprint technique for documents. The organic substances in the fingerprint residue are charred and appear as dark impressions. Although heat cannot be considered a practical latent fingerprint method, a number of arson cases have been solved as a result of latent prints developed by heat.
Such prints can be of paramount importance when processing of the evidence by regular techniques is precluded due to excessive charring. The flame technique, for softening dried impressions and producing soot that adheres to the latent marks, was used successfully in several cases in the past. Its use is quite scarce, but again, in arson cases, latent prints may be developed by soot.
Particular cases
Enhancement of bloody fingerprints Special techniques are often required for the enhancement of weak fingerprints in blood. A nondestructive optical technique has been suggested based on the reflection of fresh blood upon illumination at 400 nm (violet). The bloody prints appear as white impressions on a darker background. Ultraviolet fluorescence can also provide useful enhancement by improving the contrast on dark surfaces.
Two groups of reagents for chemical enhancement of bloody prints are used. The first contains colorless substances catalytically oxidized in the presence of hemoglobin, to form colored products. Tetramethyl-benzidine is the most common reagent in this category. It replaced the carcinogenic benzidine, the use of which was banned in the US in the early 1970s. Other substances belonging to ‘heme-reagents’ are o-tolidine, phenolphthalin and leukomalachite green. The second group contains reagents that stain proteins. Their solutions react with proteins to form colored complexes. Amido black is a typical reagent in this group. Another staining reagent is Coomassie blue. DFO is used as a fluorogenic reagent for bloody prints, especially on porous surfaces.
Fingerprints from adhesive-coated surfaces Fingerprint development on adhesive tapes is a challenge particularly in trying to solve terrorist-related cases. In 1981, a successful development with Coomassie brilliant blue 250 was reported. Since then, more sensitive staining methods have been studied; the current technique is based on staining the sebaceous components of the fingerprint deposit by a solution of gentian violet. Latent prints appear as dark purple impressions against a lightly stained background. The use of ‘Sticky Side Powder’ has also been proposed, and its use is becoming widespread.
Research and Development
Although the use of latent fingerprint detection to associate an individual with a crime scene started over a hundred years ago, systematic research in this area only began in the 1970s, with the optimization of existing techniques for fingerprint development and the search for newmethods for particular surfaces. The lag between the general scientific knowledge and the scientific back-up to fingerprint practitioners is illustrated by the history of the use of ninhydrin. Shortly after the discovery of the ninhydrin reaction with amino acids in 1910, chemists noticed that many common materials, including sweat, form blue-colored products upon reaction with ninhydrin. Since the introduction of paper chromatography in the early 1940s, ninhydrin has been routinely used to locate amino acids on chromatograms. Despite its use over the years and the oft-repeated admonition against touching chromatograms exposed to ninhy-drin, only in 1954 was ninhydrin recognized as a latent fingerprint reagent.
The application of physicochemical techniques to the visualization of latent fingerprints is one of the most dynamic and prolific areas of present research and development in forensic science. It can be divided into three categories: improvement and optimization of existing methods; the search for newreagents and techniques; and the search for solutions to problematic surfaces.
Two groups in particular have been active in optimizing fingerprint techniques (see Further Reading list for details). The main line of research at present is the search for nonpolar solvents to replace the banned freon 113, optimal working conditions for the chemical reagents (PD in particular) and the constant improvement of electro-optical devices.
Alternate light sources are becoming lighter, more powerful, more versatile and safer. Instead of the large and cumbersome 18 W argon-ion lasers, there are nowmore powerful, tunable illumination devices that emit light from ultraviolet to red, and can be operated at crime scenes. In addition, mobile argon-ion laser laboratories have been constructed, thus solving the portability problem. Specific modifications in other techniques, such as vacuum cyanoacry-late, introduction of field devices and electronic gating of optical observation systems are currently being tested. In an attempt to increase the detectability of latent fingerprints by amino acid reagents the use of proteolytic enzymes has been suggested that break down the proteins present in fingerprint deposits, to amino acids that react in a much stronger manner.
In 1973, systematic research of fingerprint powders and their adherence mechanism was started. Nearly twenty years later, the common aluminum powder was modified by increasing the content of stearic acid, thus lowering background noise and improving contrast. Powder dusting is the simplest and most widely used fingerprint technique; it still requires comprehensive research to have a better understanding of its mechanism and to improve both its sensitivity and versatility.
Modern research into newtechniques started, perhaps, in 1954 with the introduction of ninhydrin to fingerprint detection. Other milestones in this category are: the adaptation of the physical developer and vacuum metal deposition techniques; the introduction of lasers to fingerprint visualization and the secondary treatment with zinc salts; and the introduction of cyanoacrylate. Systematic research of chemical alternatives to ninhydrin was first reported in 1982 with the synthesis of several ninhydrin analogues. One of them, benzo[f]ninhydrin, showed some promise but was surpassed by other newly developed reagents. Other groups started to explore this area, and numerous such compounds have been prepared and studied. Of particular importance among them were 5-meth-oxyninhydrin and 5-methylthioninhydrin. The luminescence of their products with latent fingerprints after zinc chloride treatment was much stronger than that of ninhydrin-developed prints. The next step was the development of DFO, which has become the most important fluorogenic reagent for latent fingerprints. Current research on fluorogenic reagents focuses on substances from the 1,2-indanedione series (Fig. 5).
The search for solutions to problematic surfaces focuses on two surfaces in particular: human skin and spent cartridge cases. Fingerprint detection on human skin is a most difficult challenge since fingerprint deposits, as well as skin coating, comprise the same secretory compounds. Hence, in terms of ‘signal to noise’ ratio, all physicochemical methods give very poor results. In the fewreported successes in death investigations, the latent prints were most likely contaminated with foreign materials such as oil or grease. Several groups, however, have reported successful trials under controlled conditions. Dusting with lead powder followed by X-ray imaging has been recommended. Among the other approaches more recently studied are initial transfer to glass, plastic film, or glossy Kromekote paper and further development by traditional methods; transfer to electrostatically charged plastic sheet and examination by oblique lighting; and an iodine-silver plate technique which starts with iodine fuming of the area under examination, transfer to a polished silver plate and exposure to strong light. A brown image of the lifted print is formed on the silver plate. Prints have been developed from cadavers by this method up to 14 hours after deposition.
Cyanoacrylate fuming, followed by treatment with the fluorescent dye, TEC, gave very promising results on postautopsy cadavers. The successful use of iodine-fuming followed by fixation with naphtho-flavone has also been reported. However, attempts to apply the reported methods in actual cases gave significantly inferior results to those obtained under controlled conditions.
From critical evaluations of the various techniques it is quite clear that, for all practical purposes, there is no general solution at present to fingerprint development from human skin.
Fingerprint development from spent cartridge cases could be of great benefit in serious crime investigations. Most fingerprints, however, are destroyed during the shooting process. In 1995, it was shown that the main cause of deterioration is not high temperature as previously assumed, but the friction between the cartridge case and the chamber at the moment of ejection, which is caused by the widening of the cartridge due to high pressure during firing. Several groups have reported, however, that some portion of the fingerprint residue does remain on cartridge cases even after firing.
In 1976, latent fingerprints on spent cartridges were visualized by exposing them to nitric acid fumes and even by dusting with black powder. Positive results have also been achieved by cyanoacrylate fuming, especially on nickel cases, and by Gun-blue solution on brass, nickel and copper cases. It has been claimed that vacuum cyanoacrylate followed by fluorescent staining and treatment with selenous acid are suitable methods for fingerprint development on spent cartridges, and it has been shown that sebum-rich latent marks are much more resistant to the firing process. In some cases, they could be visualized by plating the cartridges with palladium or silver in solution or by exposing them to gold or aluminum vapors.
It is now widely accepted that the swelling stage and ejection of the firing process in semiautomatic weapons inhibits successful development of latent fingerprints on most types of ammunition. It is assumed that in those cases where positive results have been achieved, there was some tolerance between the cartridge and the chamber, so that even after the expansion, the friction was not too damaging.
