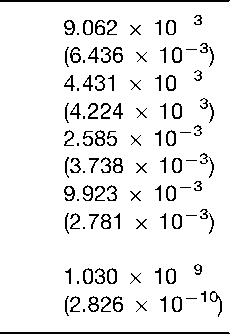Introduction
More than twenty years have passed since the technique of Southern hybridization was first used to detect restriction fragment length polymorphism (RFLP) in human DNA, and a decade since this approach was used for human identification purposes in forensic casework. The RFLP method is a direct test in which genomic DNA is isolated from biological material and analyzed without any prior amplification via the polymerase chain reaction (PCR). Briefly, DNA is extracted and cleaved into small fragments using a selected restriction endonuclease and polymorphic variation in the lengths of the resulting restriction fragments is used to distinguish between individuals. The analysis of a relatively small number (4-6) of highly polymorphic regions or loci within the human genome, commonly referred to as minisatellites or variable number of tandem repeats (VNTRs), is sufficient to generate DNA profiles that are virtually individual specific. Although the RFLP method is quickly being replaced by PCR-based typing methods, some forensic laboratories still rely heavily on RFLP typing. Moreover, RFLP typing continues to be a dominant technology for many paternity testing laboratories.
Genetic Markers Used for Forensic RFLP Typing
By far the most polymorphic sequences in the human genome are the so-called minisatellite or VNTR loci. As the name implies, VNTR loci are composed of short oligonucleotide repeat sequences which are organized into long tandem arrays. It is estimated that there are several thousand different VNTR loci in the human genome, representing a rich source of genetic variability. The length and sequence composition of the oligonucleotide repeats vary depending on the particular locus. For example, locus D1S7 is characterized by a 9 bp repeat, whereas D2S44 has a 31 bp repeat. The number of repeats in each array is highly variable and this polymorphism can be measured using a restriction endonuclease (RE) that has invariant or ‘fixed’ cleavage sites in the unique sequences immediately flanking the array. As shown in Fig. 1, the length of the resulting restriction fragment will be proportional to the number of repeats within the array.
The forensic application of RFLP typing came in 1985 when Alec Jeffreys and co-workers used the technique to generate individual-specific DNA fingerprints. This procedure uses hybridization conditions under which the VNTR probe is capable of simultaneously detecting many different VNTR loci. This approach, commonly referred to as multilocus analysis, results in complex and individual-specific hybridization patterns consisting of 8-33 bands per probe per individual. Although multilocus analysis affords a high degree of discriminating power, the complexity of the DNA fingerprints makes it difficult to delineate the possible origins of mixed samples, i.e. those containing DNA from more than one individual. In addition, multilocus analysis is not recommended for samples in which the genomic DNA is significantly degraded or limited in quantity.
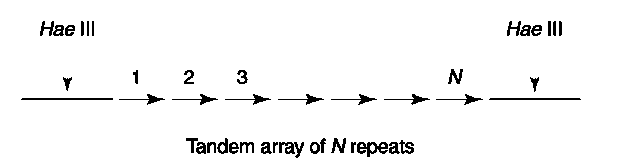
Figure 1 Schematic representation of a typical VNTR locus. The repeat sequence is such that Hae III does not cleave within it, but rather within the flanking unique sequences. Accordingly, the length of the Hae III fragment will be proportional to the number of repeats within the array.
In North America and elsewhere, forensic RFLP typing has been dominated by single-locus VNTR analysis. For this type of test, the VNTR probe is hybridized under conditions that preferentially restrict hybridization of the probe to its corresponding locus. The resulting hybridization pattern consists of one or two bands, depending on whether the individual is homozygous or heterozygous, respectively, for that particular locus. The successive typing of several independent VNTR loci enables one to assemble a multilocus DNA profile, one locus at a time (Fig. 2).
In an effort to develop standardized procedures and to facilitate the sharing of information, forensic laboratories have adopted protocols that are based on a single RE and a common panel of VNTR loci. For example, Hae Ill-based RFLP typing systems are used by the vast majority of forensic DNA typing laboratories in North America. Hae III recognizes the sequence 5′-GGCC-3′, cleaving between the second G and the first C and generating blunt-end fragments. Hue III cleaves the human genome into > 107 fragments, most of which fall into the low-molecular-weight range of less than 10 kb. Several different VNTR loci are compatible for RFLP typing with Hue III, including D1S7, D2S44, D4S139, D5S110, D10S28, D14S13, D16S85 and D17S79. It is also noteworthy that forensic RFLP programs have been established using other REs (e.g., Hinf I and Pst I) and different VNTR loci.
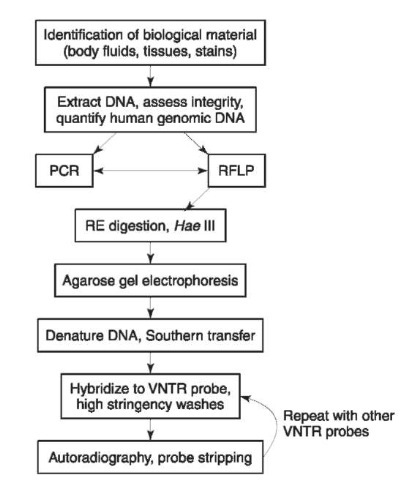
Figure 2 Schematic representation of the steps in the RFLP typing procedure using probes specific for multiple, independent VNTR loci.
In addition to VNTR loci, some forensic laboratories use probes specific for Y-chromosome sequences to determine the gender of the DNA source. One such locus, DYZ1, comprises several thousand copies of a tandemly repeated 3564 bp Hue III fragment. This fragment is detected in DNA from males (containing the X and Y sex chromosomes) but not in females (containing two copies of the X chromosome). As an internal control for the overall RFLP typing procedure, invariant or monomorphic loci can be analyzed. These are regions of DNA that yield the same pattern in all individuals, males and females alike. D7Z2 is a commonly used monomorphic locus composed of a tandemly repeated 2731 bp Hue III fragment.
The RFLP Typing Procedure Extraction of genomic DNA
As with all DNA typing procedures, RFLP analysis begins with the extraction of genomic DNA from the biological samples of interest. Generalized extraction protocols can be applied to a wide range of body tissues and fluids, deposited on even a wider range of substrates. Initially, the cellular material in the sample is solubilized and lysed in a buffered solution containing salts, detergents, and proteinase K. The detergents lyse the cells and the proteinase K digests cellular proteins, including the histone and nonhis-tone components of chromosomal DNA. The resulting cellular debris is then removed by successive extractions with organic solvents (phenol, chloroform), after which the naked genomic DNA is purified by ethanol precipitation or filter microconcentration. As an alternative to proteinase K digestion and organic extractions, cellular proteins can be precipitated with high molarity salt solutions and the DNA purified by ethanol precipitation. Yet another approach involves the use of commercially available silica-based spin columns that selectively bind the DNA while excluding proteins and other cellular debris.
A subtle, yet very important modification of the above procedures is used to isolate genomic DNA from sexual assault swabs. Postcoital vaginal swabs generally contain both seminal fluid and epithelial cells, originating from the male and female, respectively. For such samples, the general DNA isolation procedure could yield a mixture of male and female genomic DNAs. This can be avoided by simple pre-treatment of the swabto separate the intact sperm heads from other cellular material that may be present on the swab. The swab is first incubated in a solution containing detergents to lyse the vaginal epithelial cells and release the female DNA into the aqueous phase. However, since the lysis solution lacks reducing agents such as dithiothreitol (DTT), the sperm heads remain intact and can be pelleted by centrifugation. After differential separation, the sperm pellet can be processed in the presence of a reducing agent. By using such a strategy, the male (sperm) and female (vaginal epithelial) genomic DNA components of the sexual assault swabcan be isolated and analyzed separately. It should be noted that the differential extraction of sperm and vaginal epithelial DNA from sexual assault swabs is not absolute. Depending on the relative proportions of sperm and vaginal epithelial cells or the degree of sample degradation, the male and female fractions may exhibit varying degrees of crosscontamination.
Assessment of DNA integrity and quantity
The overall quality or integrity of the genomic DNA is important since it is difficult to detect high-molecular-weight alleles from samples that are significantly degraded. DNA integrity can be easily assessed by agarose gel electrophoresis of a small portion of the unknown DNA sample and control samples of known DNA concentration and molecular weight. After elec-trophoresis, the yield gel is stained with ethidium bromide and the DNA is visualized by ultraviolet light fluorescence. The presence of a discrete high-molecular-weight band (> 20 kb) on the yield gel is indicative of intact genomic DNA that may be suitable for RFLP typing. In contrast, degraded genomic DNA forms a continuum of fragment lengths (a smear), the appearance of which depends on the extent of degradation. Highly degraded samples appear as low-molecular-weight smears with little or no sequences above several kilobases in length, whereas moderately degraded samples appear as a high-molecular-weight fraction from which downward smearing is evident.
The yield gel provides a rapid and sensitive indication of DNA quantity and quality, but cannot distinguish human genomic DNA from that of nonhuman sources. Since forensic samples may be contaminated with nonhuman material (e.g. bacteria, fungi), additional tests can be used to quantify the amount of human genomic DNA in a sample. This involves slot-blotting a small aliquot of the DNA sample to the surface of a nylon membrane, followed by hybridization with a labeled probe that recognizes highly repetitive human-specific DNA sequences (e.g. alpha satellite). The quantity of human genomic DNA in a sample is then estimated by comparing the intensity of its hybridization signal to that of known amounts of human genomic DNA. Once the amount of human genomic DNA has been accurately quantified, informed decisions can be made as to the amount of DNA required for successful RFLP typing. Although the RFLP method can be used to derive DNA profiles from as little as 50 ng of human genomic DNA, most forensic RFLP typing protocols have been optimized for amounts of genomic DNA on the order of 500 ng or greater.
Forensic RFLP typing
RFLP typing of VNTR loci involves a succession of analytical procedures (Fig. 2). The first step is to cleave the genomic DNA into small fragments with a restriction endonuclease (RE) such as Hae III. The lengths of the VNTR-containing fragments are then measured by sorting the DNA fragments according to size using a technique called agarose gel electrophoresis. DNA samples are loaded into wells along one end of the gel and an electric current is applied across the length of the gel. The DNA molecules migrate through the agarose gel, with the smaller fragments travelling at faster rates than larger fragments. After electro-phoresis, the DNA fragments form an orderly array with the largest fragments located closest to the origin and a continuum of fragment lengths of decreasing sizes located at increasing distances from the origin. Each electrophoretic analysis also includes regularly spaced lanes of marker DNA fragments of known sizes. The sizing ladders are of nonhuman origin and therefore do not crosshybridize with human probes.
After electrophoretic separation, the double-stranded DNA fragments are denatured or converted into the single-stranded form by soaking the gel in an alkali solution. The fragile nature of the gel makes it unsuitable for the remaining steps in the RFLP procedure, a problem that is overcome by transferring the denatured DNA fragments from the gel to the surface of a nylon membrane (i.e. Southern transfer). The membrane-bound genomic DNA is then hybridized to a single-stranded DNA probe specific for the VNTR locus being examined. To detect the sizing ladder, labeled marker probe is also incorporated into the hybridization solution. The probes are labeled with a radioactive isotope of phosphorus (generally 32P-dATP or 32P-dCTP) or coupled to the enzyme alkaline phosphatase. After hybridization, the membrane is washed under high stringency conditions to remove any probe that may be bound nonspecifically to the membrane or to noncomplementary sequences.
Autoradiography is used to visualize the membrane-bound probe and thereby create a permanent image of the DNA profile. The membrane is over-layed with radiographic film and radioactive decay from the 32P-labeled probe (or chemiluminescence from alkaline phosphatase labeled probes) gives rise to dark bands on the developed film, coinciding with the locations where the probe was bound to the membrane. The relative position of the bands is then used to estimate the length of the tandem array present at each VNTR locus. At each locus most individuals will be heterozygous, and the DNA profile will consist of two different fragment lengths appearing as two discrete bands located at different positions along the length of the membrane. A small minority of individuals will be homozygous, having single-band profiles because they inherited fragments of similar or identical size from both parents.
After the first VNTR locus has been analyzed, the probe is stripped from the membrane and the hybridization analysis is repeated using a probe specific for another VNTR locus. This process is repeated in a sequential fashion until a panel of four or more independent VNTR loci has been analyzed. Fig. 3 shows RFLP profiles of four unrelated individuals derived using four independent VNTR loci. None of these individuals match at even a single locus, illustrating the high degree of discriminating power afforded by this type of analysis.
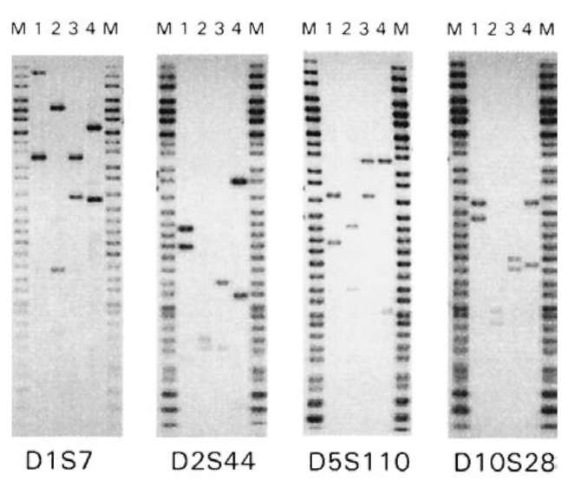
Figure 3 VNTR profiles of four unrelated individuals (lanes 1^) derived using Hae III. The molecular weight marker (lanes M) is from Life Technologies.
Interpretation of RFLP Typing Results
Image analysis
Image analysis involves the examination of the hybridization pattern on the radiographic film to estimate the sizes of the corresponding DNA fragments (Fig. 3). The rate of electrophoretic migration for DNA fragments is inversely proportional to the logarithm of the molecular weight of the fragment. Applying this equation or derivatives thereof, a relationship can be drawn between the electrophoretic migration of the fragments of the sizing ladder and their known sizes. This in turn can be applied as a relative standard to estimate the size of any genomic DNA fragment from its electrophoretic mobility. Several different computer-assisted devices have been specifically designed for making objective determinations of the electrophoretic migration of sample fragments relative to a given sizing ladder.
Defining VNTR alleles
The degree of measurement imprecision associated with RFLP typing makes it impossible to treat VNTR fragments as truly discrete alleles. In recognition of this inherent technical limitation, conservative approaches have been developed for defining VNTR alleles and assigning allele frequencies. Many forensic laboratories use the so-called fixed-bin method to define VNTR alleles, in which the length of the electrophoretic separation is subdivided into 31 arbitrary size categories with defined boundaries. The dimensions of these size categories, or fixed-bins, are such that they adequately compensate for measurement imprecision. By using this method, continuous distributions of VNTR fragments can be treated as 31 arbitrarily defined alleles.
Another approach to estimate the frequency of a given fragment length involves the use of floating-bins, whereby conservative sizing windows are centered around the estimated size of the fragment of interest. For example, the frequency of a 5000 bp fragment would take into account the observed occurrence of fragments within a window of 5000 bp + X%, where X is a value that reflects the empirically derived estimate of measurement imprecision for that particular laboratory. Floating-bins of +5% are considered to provide frequency estimates that are as conservative as those derived using the fixed-bin approach. Table 1 illustrates how the fixed-bin and floating-bin methods can be used to estimate the frequency of occurrence for a four-locus VNTR profile.
Prior to the most recent report of National Research Council, there was considerable scientific debate regarding the various methods used to estimate the frequencies of VNTR fragment lengths. However, both the fixed-bin and floating-bin (+ 5%) methods have been endorsed and continue to be used by forensic laboratories engaged in RFLP typing of
VNTR loci.
Defining a match
As mentioned above, RFLP typing of highly polymorphic VNTR loci does not provide for the unambiguous resolution of individual fragment lengths. Due to the limited resolving capacity of the overall process, identical samples analyzed in adjacent sample lanes generally may not yield the same estimated fragment sizes (e.g. lane 1 = 2500 bp, lane 2 = 2400 bp). Thus, in an absolute sense, the estimated sizes of the VNTR fragments cannot be used to define a match.
Upon visual examination of two DNA profiles,Table 1 Estimated frequencies of the four-locus DNA profile of individual no. 4 from Fig. 3. The frequencies were derived using a database for southern Ontario (Canada) and the fixed-bin and floating-bin (parentheses) methods. Floating-bins of + 5% were used for this analysis. All genotype frequencies were calculated using the formula 2pq, where p and q are the frequencies of the two observed alleles. The estimated frequency of the four-locus profile is the product of the individual genotype frequencies there are three possible conclusions that can be made: (1) the profiles are so different as to warrant an outright exclusion; (2) the profiles match and an inclusion may be justified; or (3) no conclusions can be drawn from the existing data. In addition to visual assessment of the profiles, objective match criteria have been established for forensic comparisons. These criteria are based on the size estimates for the VNTR fragment lengths and involve an empirically derived match window within which two fragment lengths must fall before they can be declared a match. For example, the Federal Bureau of Investigation uses a window of +2.5% to define the matches. Applying this match window, fragments of 2500 bp (2500 bp + 2.5% =2438-2562 bp) and 2400 bp (2400 bp + 2.5% bp = 2340-2460 bp) could be declared a match because their size ranges are overlapping.
The significance of an RFLP match
Once a DNA match has been declared and the possibility of exclusion has been discounted, it is necessary to determine how common or rare the occurrence of this matching profile is in the population of individuals who could be the source of the evidence. Specifically, the relevant question becomes: what is the probability that someone other than the accused has a DNA profile that matches the evidence? To address this question, one can simply query a relevant population database and apply conventional genetic formulae to estimate the genotype frequencies for each matching locus. The product of these individual genotype frequencies can then be used to estimate the frequency of individuals who could fortuitously match at each of the loci tested.
The Hardy-Weinberg (H-W) equation is used to estimate the genotype frequencies for each individual locus. This simple binomial equation, p2 + q2 + 2pq = 1, can be used to estimate the frequency of homozygous (p2, q2) and heterozygous (2pq) genotypes based on the observed allele frequencies (p,q). For two-band VNTR profiles, it is assumed that the bands are allelic and that the individual is heterozygous. Applying the H-W equation, the genotype frequency for a two-band VNTR profile is calculated as 2pq, where p and q are the estimated frequencies for the individual bands. For a single-band VNTR profile, it is reasonable to assume that the individual is homo-zygous and that p2 can be used to estimate the genotype frequency. However, since it is formally possible that the individual may be heterozygous for a second undetected allele, many laboratories choose to express the genotype frequencies of single-band profiles as 2p, where p is the estimated frequency of the detected allele.
Having estimated the genotype frequencies for the individual matching loci, the overall frequency for a multilocus DNA profile is calculated as the product of individual frequencies. The conservative nature of the binning procedure results in genotype frequencies for individual loci that generally are on the order of 0.01 (1/100). However, the application of the product rule across four or five loci results in overall frequencies that can be extremely discriminating (Table 1).
Strengths and weaknesses of RFLP typing
RFLP typing provides the capability to generate highly individual-specific DNA profiles through the direct analysis of only a few independent VNTR loci. Moreover, the relatively large amounts of DNA required for RFLP typing makes this method less vulnerable to contamination by DNA from extraneous sources (e.g. investigating officers or laboratory personnel). Notwithstanding the enormous impact RFLP typing has had on forensic science, there are several shortcomings associated with this methodology. First and foremost, the limited sensitivity of detection afforded by RFLP typing makes it difficult or impossible to obtain meaningful profiles from trace biological evidence or from samples that have significantly compromised due to aging or environmental insults. In addition, the interpretation of VNTR profiles can sometimes be difficult due to artifacts of the procedure such as partial Hue III digestion, band shifting, and incomplete profiles due to faint hybridization of low-molecular-weight alleles or degradation of high-molecular-weight alleles. Lastly, RFLP typing is quite time consuming, laborious and difficult to automate. Most, if not all of the inherent limitations of RFLP typing have been overcome through the development of PCR-based DNA typing systems.