Introduction
Pharmacology is the study of the effect that a drug has on biological systems. Since ‘forensic’ refers to the application of science to legal issues, ‘forensic pharmacology’ is therefore the study of drugs and their effects as they relate to the law. Examples of the types of case that may involve a forensic pharmacologist would include those involving the effect of medications and/or illicit substances on the mental and physical state of a victim or perpetrator of a violent crime; driving under the influence of alcohol or drugs; malicious or accidental poisoning; adverse drug reactions and interactions; and competency to stand trial.
The pharmacologic effect of a drug is associated with both the rate and concentration at which it reaches its site of action. These parameters in turn depend upon the absorption, distribution and elimination of the drug by the body. Before we can assess the effect that a drug has on a particular individual, we must therefore consider the relationship between dose, route of administration, metabolism and the resulting concentration of the drug in the body. Pharmacokinetics is the mathematical discipline that encompasses these processes and relates the dose of the drug administered to blood concentration, and therefore pharmacological effect.
Pharmacokinetics Absorption
In order to produce an effect, the drug must enter the bloodstream and be carried to its site of action. The drug must therefore be absorbed across the gastrointestinal (GI) tract if given orally, from an injection site (unless given intravenously), across the skin (creams and ointments) or via the lungs (inhalations). The rate at which a drug enters the bloodstream will determine its onset of action. For example, intravenous administration results in an immediate effect because the drug is placed directly into the blood, whereas oral administration results in a gradual increase in blood concentration while the drug is absorbed across the Gl-tract into the bloodstream (Fig. 1).
Drug absorption from the gastrointestinal tract The majority of drugs are administered orally and are absorbed via the GI tract. There are a number of factors that can influence the rate and extent of appearance of intact drug into the systemic circulation after oral administration of a pharmaceutical. The steps involved in the release and absorption of a drug from a tablet are shown below. The rate and extent of appearance of intact drug in the systemic circulation depend on a series of processes. The slowest step in this series (rate-limiting step) will control the overall rate and therefore the onset of pharmacological effect. Rate-limiting steps vary for different drugs and include the rate of release of the drug from the particular dosage form, the solubility of the drug, its absorption across membranes, the rate of gastric emptying and the rate of metabolism.
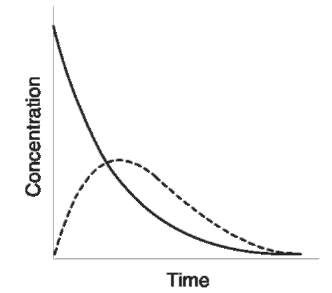
Figure 1 Plasma concentration/time curves for a drug administered intravenously (—) and orally (—).
Structure of the gastrointestinal tract The GI tract consists of three major regions: the stomach, the small intestine and the large intestine. As a drug passes through these regions, it experiences changes in pH, enzymes, electrolytes and surface features, all of which can influence drug absorption. The stomach mucosa contains many folds, which increase the surface area available for absorption. Although the stomach does not function primarily as an absorptive organ, its high blood supply and the fact that a drug can potentially reside in the stomach for several hours provide conditions suitable for the absorption of primarily acidic drugs. The small intestine, or ileum, is the most important site for drug absorption in the GI tract. The extremely large surface area of the small intestine results from the existence of folds in the intestinal mucosa, villi and microvilli. Most drugs reach the systemic circulation via the bloodstream of the capillary network in the villi. However, it is possible that the absorption of highly lipid-soluble drugs may occur via fat absorption pathways. The colon or large intestine, like the stomach, lacks the villi and microvilli of the small intestine, but the large intestine serves as a site for the absorption of a drug that has not been completely absorbed in the small intestine.
Mechanisms of drug transport across the gastrointestinal barrier The epithelium lining the GI tract is considered to constitute the main cellular barrier to the absorption of drugs from the GI tract. Submicroscopic, aqueous channels or pores penetrate the lipid membrane. Water-soluble substances of small molecular size, such as urea, are absorbed by simple diffusion through these channels. The majority of drugs cross the membrane by passive diffusion, whereby the physicochemical properties of the drug, the nature of the membrane and the concentration gradient determine the rate of drug transport across the membrane. The process of passive diffusion involves the partition of the drug between the aqueous intestinal contents and the membrane. Drugs entering the bloodstream will be carried away from the site of absorption by the GI blood supply and will become diluted by distribution in a large volume of blood, distribution into body tissue, metabolism and excretion, and protein-binding in the blood. Most drugs are absorbed from the GI tract by passive diffusion; however, a few lipid-insoluble compounds, such as 5-fluorouracil, are absorbed by active transport. A carrier, which may be an enzyme or other component of the cell membrane, is responsible for effecting transfer of the drug by formation of a complex. There are several active transport systems in the intestinal tract responsible for the absorption of amino acids. Drugs which resemble these compounds will also be absorbed by the same mechanism, e.g. L-dopa, which is structurally similar to tyrosine and phenylalanine, and various vitamins and sugars.
Physiological factors influencing drug absorption A number of factors affect the absorption of a drug from the GI tract. These include:
• Surface area of the absorption site: drugs are generally absorbed more readily from the small intestine because of the extremely large surface area of this structure.
• pH of the GI fluids: the acid or base environment of the GI tract will influence the degree of ionization of the drug as it passes from the stomach to the intestine. Generally, the unionized fraction of the drug is more readily absorbed by the intestinal mucosa.
• Gastric-emptying rate: most drugs are absorbed from the small intestine; hence, any reduction in the rate at which the drug leaves the stomach and enters the small intestine may inhibit or delay absorption. Gastric emptying is particularly important for drugs that are susceptible to acid or enzyme hydrolysis in the stomach. Hunger, anxiety, body position and intake of liquids can stimulate the gastric-emptying rate. Fatty foods, high-bulk diet, depression and various drugs, including alcohol, retard it.
• Intestinal motility: most drugs are designed to be absorbed after entering the small intestine, and ideally the drug should remain in this environment for as long as possible. It normally takes 3-10 h for a drug to be carried along the length of the small intestine.
• Drug stability in the GI tract: acid-base or enzymatic hydrolysis in the GI tract can reduce the bioavailability of the drug, i.e. the amount of drug available for absorption.
• Complexation of the drug with dietary components: the GI absorption of some drugs, for
example tetracycline, is reduced if taken together with dairy products, owing to the formation of an insoluble complex with calcium. Various disease states such as gastric ulcers can also affect the degree of drug absorption from the intestinal tract.
Physicochemical factors affecting drug absorption
In addition to physiological factors, the physical chemistry of the particular drug also determines its rate and degree of absorption from the intestine. The dissolution rate of a drug is important because it can only be absorbed from the GI tract once it has dissolved. Dissolution rate is affected by the particle size, the presence of polymorphic forms, salts and other excipients. In general, tablets take longer to dissolve than capsules or suspensions. The greater the time taken for the drug to dissolve, the slower the onset of action. The dissociation constant (piCJofthe drug determines its extent of ionization in the stomach and intestine and therefore the extent of absorption. The octanol/water partition coefficient of the drug gives an indication of the extent of drug absorption from the GI contents to the mucosa.
Distribution
Distribution of a drug to the tissues is subject to the same processes as absorption and is affected by the route of administration, the protein-binding of the drug, the blood supply to various organs, and the rate of metabolism and excretion of the drug. An indicator of distribution is the volume of distribution, which is determined by dividing the amount of drug in the body by the plasma concentration. If the volume of distribution for a particular drug is known, then the total amount of that drug can be determined from an analysis of the plasma concentration.
Elimination
Drugs are eliminated from the body primarily by metabolism by the liver to a more polar compound, followed by excretion in the urine by the kidneys. The rate at which a drug is eliminated from the body is described by the term clearance. Clearance generally refers to the volume of plasma from which a drug is completely removed per unit time. Plasma half-life is the time taken for plasma drug concentrations to decline by 50%. Both clearance and plasma half-life vary greatly between different drugs, and even between individuals.
Drug metabolism
Metabolism is an integral part of drug elimination. As well as facilitating excretion of the drug, it may also affect the pharmacological response of a drug by altering its potency and/or duration of action. When a polar (or ionized) water-soluble drug is absorbed in the body, it is largely excreted unchanged by the kidneys. However, the majority of drugs are lipid-soluble to some extent. Such compounds must undergo extensive metabolism, and are converted into a more polar, water-soluble form before they can be excreted in the urine. Although metabolism is generally considered to be a detoxification process, some of the metabolic products may also have pharmacological activity and may be toxic. Examples include aspirin, which is hydroxylated to salicylic acid; diazepam, which may be hydrolyzed to temazepam and oxidized to oxazepam; and amitriptyline, which is demethylated to nortriptyline.
Pathways of drug metabolism can be nonsynthetic
- for example, oxidation and hydrolysis – or synthetic
- for example, conjugation with glucuronic acid or sulfate. Despite the extensive range of reactions that a drug molecule may undergo, the majority are catalyzed by membrane-bound enzymes in the liver cells (hepatocytes). For example, the cytochrome P450 mixed-function oxidase system catalyzes oxidations and conjugation with glucuronide. Metabolism can occur in the plasma as well as organs other than the liver, such as the GI tract, kidneys and lungs.
Oxidation Metabolic oxidation reactions include N-demethylation, e.g. diazepam — nordiazepam (desmethyldiazepam); hydroxylation, e.g. diazepam- temazepam; N-or S-oxidation, e.g. chlorproma-zine — chlorpromazine N-oxide or chlorpromazine sulfoxide.
Hydrolysis Hydrolysis is an important route of metabolism for esters and amides, e.g. aspirin — salicylic acid. This may be as a result of acid, base or water hydrolysis, or due to esterases in the plasma or liver.
Reduction reactions Aldehydes and ketones may undergo reduction to primary and secondary alcohols, e.g. prednisone — prednisolone. Nitro compounds (NO2) are reduced to amines (NH2).
Conjugation reactions Oxidation, reduction and hydrolysis usually produce metabolites, which have a reactive functional group. Several of the more common conjugation reactions that mask these functional groups include glucuronide or sulfate formation, acetylation and methylation. Glucuronic acid can be conjugated with a wide range of functional groups to form either an ester (as with carboxylic acids) or ether with phenols and alcohols. N-or S- glucuronides may be produced as well as O-glucuronides, all of which facilitate drug elimination by the kidney. Sulfate conjugates are formed with alcohols and phenols. These are salts of strong acids and are readily excreted in the urine. Acetylation under the influence of the enzyme N-acetyltransferase occurs frequently with primary and secondary amines, and methylation is a common feature of the metabolism of phenols.
Factors affecting metabolism
Similar drug concentrations will not produce the same pharmacological effects in all subjects. These differences may be due to one of the following factors.
Age Young children and elderly people generally have a lower metabolic capacity compared with that of subjects between these extremes of age. The enhanced sensitivity of the very young to drugs can be accounted for by the fact that the microsomal enzymes, which are responsible for metabolism (particularly conjugation), are not fully active until several months after birth. Older children (5 years) metabolize drugs at a similar rate to adults. The dose must be lower, however, to take account of the smaller volume.
In elderly patients (over 60 years) there is a decreasing capacity for drug metabolism, as a consequence of a gradual decline in physiological efficiency. In addition, the amount of protein-binding may decrease and renal excretion may be reduced. Elderly people may therefore experience higher blood levels of a drug compared with younger patients.
Disease Diseases can affect all of the processes by which a drug is absorbed, distributed and eliminated from the body. A drug may be poorly absorbed during GI disturbances. The rate at which drugs cross tissue membranes can be altered in cardiovascular disease, which may alter peripheral blood flow. Endogenous free fatty acids released into the plasma during trauma can displace drugs that are weak acids from albumin-binding sites.
Diseases that affect the liver and/or kidneys probably have the greatest effect on drug concentrations, having a direct effect on metabolism and excretion. If liver function is impaired as a result of disease or chronic drug use, blood levels will be greatly increased.
Weight The weight of a patient affects drug concentrations in the blood, as it determines the volume into which the drug is distributed. Drug metabolism tends to be faster in males than in females.
Genetic factors The genetic control of the number of receptor sites and genetic variations in the extent of protein-binding or the rate and extent of drug metabolism can make a marked contribution to variations in drug concentrations and responses. The inability to oxidize tolbutamide, for example, has been related to an increased incidence of cardiovascular toxicity of the drug.
Diet Diet has been demonstrated to influence the metabolism of some drugs; for example, the conversion of an asthmatic patient from a high-protein to a low-protein diet will increase the half-life of theophylline. The exposure of the patient to other drugs (particularly alcohol) can have a marked effect on the metabolism of certain drugs.
Excretion of drugs
The excretion of drugs and metabolites terminates their activity and presence in the body. They may be eliminated by various routes, with the kidney playing the major role with excretion into the urine. Drugs may also be excreted in the feces, bile, lungs, sweat, saliva and breast milk. Some drugs may be reab-sorbed from the renal tubule even after having been sent there for excretion. Because the rate of reabsorp-tion is proportional to the concentration of drug in the unionized form, it is possible to modify this rate by changing the pH of the urine. For example, acidification of the urine with citric acid causes reabsorp-tion of acidic drugs, whereas making the urine alkaline with sodium bicarbonate causes reabsorp-tion of basic drugs.
Pharmacology
The major classes of drugs that possess abuse potential can be classified as sedative hypnotics, stimulants and hallucinogens.
Sedative hypnotics
Opium has been used as a sedative since ancient Greek times. It is a natural product obtained from the dried latex of the opium poppy Papaver somniferum. Crude opium is a rich source of the narcotic analgesics codeine and morphine. It has long been known that inhalation of the vapors produced by heating opium will cause sedation and a long peaceful sleep. Unfortunately, addiction to the drug develops rapidly. Opium is still occasionally found as a street drug, although presently the majority of opiate abuse is associated with heroin, a semisynthetic derivative of morphine. Heroin has similar actions to morphine, and indeed opium, but is a more powerful analgesic and is also more addictive. Illicit heroin is either injected intravenously or smoked by inhalation of vapors produced by heating a small amount of the drug on aluminum foil. The presence of adulterants, such as quinine, strychnine and various cutting agents, including sugar, starch and even household cleaner and brick dust, make intravenous administration of this and other illicit preparations extremely hazardous.
Absorption The opiates are absorbed rapidly after parenteral administration. Oral absorption of morphine is variable and results in a bioavailability of only around 20%. It is distributed primarily in the kidneys, liver, lungs and spleen but does not accumulate in the tissues. The major metabolism of morphine is by conjugation with glucuronide to form morphine 3- and 6-glucuronides. N-demethylation, O-methyla-tion and N-oxide formation are minor metabolic pathways. The half-life of morphine in the plasma is approximately 3 h. After parenteral administration, approximately 10% of the dose is excreted as the unchanged drug and about 70% as glucuronides.
Heroin, or diacetylmorphine, is rapidly hydrolyzed in the blood to 6-monoacetylmorphine and then to morphine. The excretion of heroin in the urine is similar to that of morphine, with the majority being converted to morphine glucuronide. The detection of low concentrations of 6-monoacetylmorphine is an indication of the use of heroin as opposed to morphine. Codeine is rapidly absorbed following oral ingestion. It is then metabolized primarily to co-deine-6-glucuronide, with some demethylation to morphine and, in turn, morphine glucuronides.
Effects The pharmacological effects of the opiates are all similar, exerting their main effects by action on the central nervous system. The opiates are most effective in the treatment of moderate to severe pain. Behavioral changes induced by these drugs vary and can result in euphoria or nervousness and fear. Depression of the central nervous system (CNS) can result in respiratory depression, and respiratory failure is a common cause of death in cases of opiate overdose. Pinpoint pupils and constipation are common indicators of opiate use. Tolerance to opiates develops quickly. This is not due to an increase in metabolism but to deactivation of the opiate receptors. On withdrawal, the receptors become supersensitive and the system becomes overactive. The resulting withdrawal symptoms include exaggerated yawning and shivering and profuse sweating. Severe diarrhea and vomiting, resulting in significant weight loss, can in some cases be fatal. Goose flesh and involuntary muscle twitches have resulted in the expressions ‘cold turkey’ and ‘kicking the habit’ when referring to heroin withdrawal.
Methadone, a synthetic opiate, is widely used in the treatment of opiate addiction. Methadone merely substitutes for the heroin. Its longer half-life and the fact that it is administered orally rather than intravenously mean that it has less abuse potential than heroin. The effects of opiate intoxication can be completely blocked or reversed in an emergency situation by use of an opiate antagonist such as naloxone. Administration of such a drug will, however, immediately send the patient into withdrawal.
Other sedatives
Barbiturates are the prototype drug in this class, although they are no longer widely used, having largely been replaced with the benzodiazepines. Abuse, addiction, tolerance and dependence develop with all members of this class. The historical development of sedatives has seen the introduction of one drug after another, each with its own abuse potential. Unfortunately, it is impossible to treat anxiety, depression and insomnia using current drug therapy without the risk of addiction. The nature of the symptoms of these conditions, and the fact that drugs act directly on the CNS can lead to abuse and dependence. The barbiturates are derivatives of barbituric acid; in general, those compounds possessing substituent groups that confer greater lipid solubility have the faster onset of action and shorter half-life. Barbiturates may be administered orally or intravenously. Most are extensively metabolized, with less than 5% of a dose of the more lipid-soluble compounds amobarbital and pen-tobarbital being excreted unchanged. Phenobarbital, which is the most polar of the common barbiturates, is excreted as 25% unchanged in the urine. Metabolism of the barbiturates typically involves hydroxyla-tion, oxidation or dealkylation. All of these compounds have half-lives greater than 24 h, which can result in accumulation in the body with repeated doses. Even after a single dose, barbiturates and/or metabolites are excreted in the urine for several days.
Benzodiazepines The benzodiazepines have largely replaced the barbiturates for the treatment of anxiety and are available for both oral and intravenous administration. They are probably the most over-prescribed group of drugs in use today. Chlordiazep-oxide, oxazepam, lorazepam and alprazolam are absorbed from the GI tract relatively slowly, and peak plasma concentrations may not be reached for several hours. Diazepam is rapidly absorbed and peak concentrations may be achieved in less than 1 h. The benzodiazepines and their metabolites bind to plasma proteins. Diazepam, which possesses high lipid solubility, is 99% protein-bound. Due to the lipophilic nature of these compounds, there is a rapid uptake into brain tissues. The benzodiazepines undergo extensive metabolism by the same routes as the barbiturates. In many cases, however, metabolism of the benzodiazepines results in the formation of other biologically active compounds. Examples include the metabolism of diazepam to temazepam and oxaze-pam. Plasma half-lives vary greatly, depending on the individual benzodiazepine. Flurazepam, for example, has a half-life of approximately 3 h, whereas the half-life of nitrazepam is around 24 h.
The sedative hypnotics depress the activity of all excitable tissue, particularly nerve cells. In large doses, the drugs can suppress function in cardiovascular activity. Early effects include loss of attention and concentration, impaired short-term memory and lack of coordination. Sedative hypnotics also cause withdrawal symptoms when their use is discontinued. These vary in severity, with the shorter-acting drugs typically having an earlier onset and more pronounced withdrawal effect. Symptoms include anxiety, muscle weakness and shaking. This may be followed by convulsions and psychoses, which can last for several weeks unless treated. Treatment is required to prevent morbidity from adverse effects, and the sedative is usually substituted with a long-acting benzodiazepine, such as chlordiazepoxide or diazepam, with fewer side effects. The dose of this drug is then reduced gradually over time.
Side effects include CNS depression, sedation, drowsiness, hostility, irritability and disturbing dreams. Weight gain, skin rash and headaches are also commonly encountered. Benzodiazepines are considered ‘safe’ in the sense that it is difficult, if not impossible, to overdose on them; however, benzodia-zepine addiction is widespread and fits of depression accompanying its use often lead to suicide attempts. Treatment consists of switching to a longer-acting benzodiazepine that causes less pronounced withdrawal effects, and gradually reducing the dose.
Various drugs act synergistically with sedative hypnotics to produce dangerous respiratory depression and cardiac failure. Ethanol, antihistamines and monoamine oxidase inhibitors can all increase the CNS depressant activity of the sedative hypnotics. In addition, the metabolism of some drugs, such as anticoagulants, digoxin and p blockers, can be enhanced as a result of the induction of microsomal enzymes by the sedatives. This effect, if not anticipated, is potentially life-threatening.
Stimulants
The most commonly abused drugs in this group are cocaine and amphetamine. Cocaine is a natural product present in the leaves of the coca bush, which is indigenous to the Andes mountain region of South America. Like opium, cocaine has a long history of use and abuse, and is still chewed by South American Indians for its stimulant effects. Illicit cocaine may be found in two forms: the hydrochloride salt, and the free base or ‘crack’. The hydrochloride is usually snorted up the nose with absorption through the mucous membranes, whereas the free base is smoked and absorbed through the lungs. The bioavailability of cocaine varies depending on its route of administration, with only 20-40% of an oral or intranasal dose being absorbed compared with nearly 100% via the lungs. Insufflation results in a euphoric high after about 30 min, compared with 75 min after an oral dose and 8 s by smoking. Cocaine is primarily metabolized by hydrolysis to benzoylecgonine, and by the action of esterases to ecgonine methyl ester. N-demethylation of cocaine results in the formation of norcocaine, which retains biological activity. Cocaine has a plasma half-life of around 40 min, and benzoylecgonine of about 90 min.
Cocaine acts by activation of the brain’s pleasure centers, which are dependent on dopamine neuro-transmission. It blocks the reuptake of dopamine from the synapse, which results in euphoria, increased motor activity and psychotic symptoms. Apart from its stimulant effects, cocaine also causes increased heart rate, cardiac arrhythmias and peripheral vaso-constriction. Consequently, sudden changes in blood pressure may result in death due to cardiac failure and cerebral hemorrhage. Cocaine addiction is treated with antidepressants, such as bromocriptine, or tri-cyclics, such as desipramine.
Amphetamine Amphetamines are generally used as oral preparations, resulting in a widespread distribution of the drug. Amphetamine undergoes several routes of metabolism: hydroxylation, N-dealkyla-tion, oxidative deamination, N-oxidation and conjugation of the nitrogen. Approximately 50% of the drug is excreted unchanged in the urine. For most amphetamines, the major route of metabolism is oxidative deamination. Unlike most drugs, chronic administration of amphetamine inhibits its metabolism, resulting in an increase in the amount excreted unchanged. The half-life of amphetamine in the blood is dependent on urinary pH, and is approximately 4-8 h if the urine is acidic and about 12 h in individuals whose urinary pH is not controlled.
Amphetamine is similar in its pharmacological effects to cocaine, and is also thought to act via its interaction with dopamine. Amphetamine promotes the release of dopamine and norepinephrine from presynaptic neurons and blocks the uptake of catecholamines. In common with cocaine, amphetamine causes restlessness, stimulation, appetite suppression, paranoia and psychosis. Other stimulants with amphetamine-like activity include methamphe-tamine, methylenedeoxyamphetamine (MDA) and methylenedeoxymethamphetamine (MDMA). As little as 3 h after cessation of chronic amphetamine use, the patient falls asleep. Sleep may last for several days and is followed by increase in appetite, anxiety, depression, irritability and lethargy. Psychosis, including paranoia, hallucinations and suicidal tendencies, may develop, from which it may take years to fully recover.
Hallucinogens
Cannabis, or marijuana, is the most commonly abused psychoactive substance. The fruiting and flowering tops and the leaf material of the Cannabis sativa plant contain the highest concentrations of the active compounds, or cannabinoids. Dried plant material may be mixed with tobacco and smoked. Hashish is a resinous extract of the cannabis plant, which is similarly smoked and contains higher concentrations of cannabinoids. Cannabis has a rapid onset of action, causing sedation and disruption of space-time perception. Although smoking is the most common route of administration, it also has some activity if taken orally. Smoking results in onset of drug action within seconds, with peak tetrahydro-cannabinol (THC) concentrations occurring after 7-10 min and declining over 2-3 h. THC is highly lipophillic and is stored in body fat, which may result in its being detected in the urine of chronic users for several weeks after the last dose. THC undergoes rapid metabolism to 8,11-dihydroxy-THC and an active metabolite 11-hydroxy-THC, which is further metabolized to A9-THC acid and excreted in the urine. The half-life of THC in the plasma is about 20-36h.
Cannabis causes increases in heart rate and peripheral blood flow, and bronchodilation. Other effects include increased body temperature, slower EEG activity, decreased intraocular pressure, increased appetite, drowsiness, visual distortions, diminished concentration and attention span, and decreased coordination. Chronic use is reported to result in mutagenic effects, as well as causing a reversible inhibition of the immune system. Psychotic delusions and paranoia may also occur with prolonged use. A common effect of chronic cannabis use is depression, which may be severe and result in suicide attempts. Anxiety and sexual dysfunction have also been associated with its use. ‘Amotivational syndrome’ is a controversial term used to describe personality changes associated with chronic use of cannabis. It is defined as a lack of interest in work or productivity.
Lysergic acid diethylamide (LSD) LSDis a semisynthetic substance produced from ergot alkaloids. It was originally developed as a possible treatment for schizophrenia, but its side effects limited its usefulness. It is one of the most powerful mind-affecting substances known – a typical dose as low as 50 ug being capable of producing an effect that will last for several hours. LSDis well absorbed after oral dose, with psychological effects becoming apparent after 45 min and peaking at 3 h after administration. Effects may last for 8-12 h. The half-life in plasma is approximately 2 h. LSDundergoes extensive metabolism in the liver, with only about 1% being excreted unchanged. 2-Oxylysergide is a major metabolite, with 13-hydroxylysergide and 14-hydroxylysergide also being produced.
LSDis thought to produce its effect by interacting with serotonin in the brain. The action mixes up the senses, commonly known as a ‘trip’. Vivid hallucinations, such as seeing music as colors, are common. Although it is thought to be impossible to overdose on LSD, deaths attributed to accidents while individuals are under the influence of the drug are relatively common. Flashbacks or the recurrence of hallucinogenic effects are also a symptom associated with LSD. They are probably due to a very small amount of the drug being absorbed by the brain tissue and later released into the system, causing a second hallucinogenic effect.
Psilocin and psilocybin Psilocin and psilocybin are naturally occurring hallucinogens present in the Psi-locybe genus of mushrooms. They have an action similar to that of LSDbut are less active. Ten to fifteen mushrooms (about 2 cm in height) will induce a hallucinogenic effect when taken orally. Psilocybe mushrooms can also be dried and smoked with tobacco or the drug may be extracted with boiling water to produce an infusion. Hallucinogenic effects generally become apparent within 30 min of ingestion. Psilocybin is readily absorbed from the GI tract, and is metabolized by dephosphorylation to the hallucinogen psilocin. The duration of hallucinogenic effect is several hours after a single dose.
Phencyclidine (phenylcyclohexylpiperidine, PCP)
Phencyclidine was originally marketed as a veterinary anesthetic. It is used illicitly as a white powder, which is taken orally, smoked together with marijuana or inhaled in combination with cocaine. It may also be injected intravenously. PCP’s onset of action accordingly varies from a few seconds with intravenous use and smoking, to 20 min after oral ingestion. The half-life is variable and is reported to range from 10 to 96 h, with effects reported as lasting between a few hours to days. Like amphetamine, the rate of elimination depends on urinary pH. Metabolism occurs primarily in the liver, with glucuronidation and hydroxylation being the principal pathways. Approximately 50% of the administered dose is excreted unchanged.
Symptoms appear rapidly and resemble schizophrenia. Users are a risk to themselves and to others when under the influence of this drug. Euphoria, depression, agitation, violence, hallucinations, paranoia, panic and suicidal tendencies are common effects. PCP users also experience increased strength and a decreased sense of pain, making them extremely dangerous and difficult to control. Eventually they lose consciousness and go into a coma. Hypertension and tachycardia are also associated with the use of PCP, which may result in cardiac failure. PCP-induced psychosis can last for up to 4 weeks, during which time the addict has to be kept sedated because of violent behavior. Phencyclidine is thought to act by increasing glucose utilization in the brain. This is probably initiated via a receptor and may involve acetylcholine. PCP intoxication is characterized by reddening of the skin, enlarged pupils, delusions, amnesia, nystagmus, excitement, arrhythmias, paranoid psychosis and violent behavior. With high doses, convulsions lead to death.
Solvent abuse
Teenagers who are unable to obtain other drugs or alcohol frequently abuse solvents. Most solvents cause CNS depression, sedation, intoxication, euphoria and hallucinations. The solvent is placed in a plastic bag and sniffed, or a cloth soaked in the solvent is placed over the nose and mouth and inhaled. Toluene is the main solvent that is abused. It causes rapid CNS depression that mimics the effects of narcotics such as morphine. It also causes liver damage and renal and cardiac failure, which may lead to convulsions and death. Solvent intoxication is initially similar to the effects of alcohol, with excitation, euphoria, nausea and vomiting. Further inhalation results in progressive depression of the CNS, accompanied by disorientation and confusion. With continued exposure, ataxia, stupor, seizure and cardiorespiratory arrest follow.
Sources of solvents include adhesives (benzene, toluene, xylene, hexane and heptane), aerosol sprays (ethanol, isopropanol, toluene and xylene), antifreeze (glycols, methanol and isopropanol), nail polish (acetone, acetates and alcohol) and glue (petroleum distillates, ethyl acetate and toluene). The inhalation of most solvents results in effects similar to those described for toluene. In addition, methanol results in abdominal cramps, headache and muscular weakness. Methanol is metabolized to formaldehyde, which causes damage to the optic nerve. Isopropanol initially causes irritation of the eyes and nose but can be fatal in sufficient doses. Butyl and amyl nitrate cause ischemia and hypotension and may result in convulsions and cardiovascular collapse. Inhalation of gasoline results in euphoric effects in less than 5 min, but symptoms may persist for up to 6 h. Cardiac arrhythmias, hallucinations and neurological problems are typical. Numerous cases of sudden death have been associated with solvent abuse. This is largely due to CNS depression followed by respiratory collapse.
