Introduction
There are two main types of forensic hair examinations: hair identification and hair comparison. Hair identification involves classifying a hair as being a member of a specific group, e.g. human pubic hair. In hair comparison, on the other hand, a determination is made as to whether or not hairs of the same group are consistent with having had a common origin. Hair identification should be considered first since it is a necessary preliminary step in hair comparison.
Elements of Hair Identification
Six elements comprise the process of hair identification:
• the questioned hair;
• a standard reference collection;
• literature references including published photomicrographs and keys;
• the equipment used;
• the list of hair characteristics;
• the examiner.
The hair to be identified is termed the questioned hair. Unless there is some logical justification for grouping them together, all questioned hairs must be considered individually.
A good authenticated standard reference collection is a most important element in forensic hair identification. Such collections, which should contain a large number of hair samples of different types and origins, can be built up through contacts with zoos and exchanges with other forensic scientists, or can be purchased commercially.
The next element in forensic hair identification is literature references. These include published photomicrographs and keys, which can supplement but not replace standard reference collections. Some of the citations in the bibliography provide published photomicrographs and keys.
The most important piece of equipment for hair identification is the comparison microscope. This consists of two compound microscopes joined by a common bridge. Using a mounting medium with refractive index of about 1.52, the questioned hair is mounted on a glass microscope slide and placed on the stage of one microscope. A microscope slide of a standard sample is placed on the stage of the other microscope. Both samples can then be viewed simultaneously in a split field. Comparison bridges also have an adjustment enabling either microscope to be used separately, if so desired. In forensic hair identification, magnifications of from 40 x to 400 x are generally used. Forensic hair examiners should be aware of the basic principles of microscopy.
Another useful piece of equipment for hair identification is a scanning electron microscope (SEM). This can be used to view hair scale patterns, cross-sectional shapes and medullary structures. Lack of access to an expensive piece of equipment like an SEM need not be a deterrent to those involved in hair identification. As will be seen in the following discussion, by means of some special techniques, the same information can be obtained using a light microscope and some inexpensive equipment.
Cast impressions of hair scale patterns can be produced by many different methods. The following method produces good quality scale impressions fairly quickly.
• Prepare a mixture of three parts colorless nail polish and one part isoamyl acetate.
• Place two or three drops of this mixture on a clean glass microscope slide and spread evenly, using the end of a second slide.
• Place a clean hair in the wet mixture.
• Dry for 10 min at room temperature, then remove the hair.
• The scale impression is now complete and ready for microscopical examination.
• The hair may then be washed in iso-amyl acetate and used for further examination.
A simpler method has been proposed whereby scale impressions can be seen on hairs fixed to glass microscope slides with clear cellulose tape. This method has the advantage of also revealing medullary structure.
Many methods have also been proposed for producing cross-sections of hairs. The recommended method uses a Schwartz fiber microtome. The hairs are first folded over several times so that cross-sections can be obtained from as many different areas along the shaft as possible. The bundle of hair is then packed with absorbent cotton and fitted tightly into the aperture on the top plate of the microtome. After trimming the bundle and assembling the microtome, the drum is turned so as to raise the bundle by about 50 um. A small dab of collodion-like solution called Micolac is then applied to the top of the hair bundle and allowed to dry for about 5 min. The section is cut in one even motion with a single edged razor blade. With a little practice, this method can fairly quickly produce good quality cross-sections.
By examining longitudinally split hairs by SEM, medullary microstructure has been shown to greatly assist in animal hair identification. However, the same information can be obtained with a light microscope by hair splitting, followed by clearing the medulla with glycerine-alcohol and examination with an oil-immersion objective.
The fifth element of hair identification, the list of characteristics, arises from the basic structure of the hair. These characteristics are listed in the Appendix.
The final and most important element in hair identification is the examiner, whose role is to synthesize all the information gained from the various characteristics and develop a pattern. This requires a considerable amount of training and experience. There are no hard and fast rules. In the following discussion many qualifying phrases such as ‘generally’ or ‘usually are’ are used. The examiner must always be aware of exceptions. No one characteristic by itself is the most important in establishing identification. Rather, the general pattern of characteristics is important. In hair identification, an examiner should strive to maximize information content while maintaining an acceptable level of possible errors, thereby arriving at conclusions that are neither understated nor overstated.
Human or Animal?
With hair identification, it can first be determined whether a hair is human (Fig. 1) or animal (Fig. 2): The following are some of the distinguishing features.
Length: Human scalp hairs are longer than most animal hairs.
Diameter: Human hairs are usually in the range of 0.05-0.15 mm; animal hairs can be narrower or coarser.

Figure 1 A human scalp hair (400 x).
Color: Animal hair can have a banded appearance; untreated human hair never does. Treatment: Except when used in textile materials, animal hair rarely exhibits dying, bleaching or other cosmetic treatment.
Medulla: Animal hairs can have a complex regular/ geometric cellular medulla; human hairs have only an amorphous medullary structure. Medullary index: Defined as the ratio of the diameter of the medulla to the diameter of the hair, the medullary index in human hair is almost always less than 1/3; in animal hair it is usually greater than 1/3. Pigment distribution: Animal hairs can have pigment distributed about the medulla whereas this is extremely rare in humans.
Shaft: Animal hairs can have different shapes than human, e.g. the spatulate shape found in rodent hairs. Root: Animal hairs have different root shapes, typically brush-like, whereas humans’ are bulb or ribbon-shaped.
Tip: Human hair (particularly scalp) is usually cut or frayed at the tip; animal hair is generally naturally tapered.
Scales: Human hair only exhibits irregular annular scale patterns; animal hair has a variety of types and can have more than one type in the same hair. Cross-section: Animal hairs can have some unusual shapes, e.g. dog bone (rabbit) and cigar shaped (seal).
Animal Hair Identification
The claim is often made in the literature that macroscopic and microscopic hair examination can usually result in species identification. It should be noted, however, that most of these topics were written by nonforensic scientists. The consequences of error on the part of a forensic scientist are much greater than, for example, with someone doing wildlife habitat studies. Forensic scientists are also acting under the constraint that, unless found in groups, all questioned hairs must be considered singly. When this is added to the large variation in characteristics of hairs taken from different locations on the same animal and the wide animal to animal variation that can occur within a species (particularly in species spread over large geographical areas), it can be seen that forensic scientists should only macroscopically and microscopically identify species of animal hairs when good complete dorsal guard hairs are present and the examiner feels confident of the identification. In all other instances, only the animal family of origin should be identified.
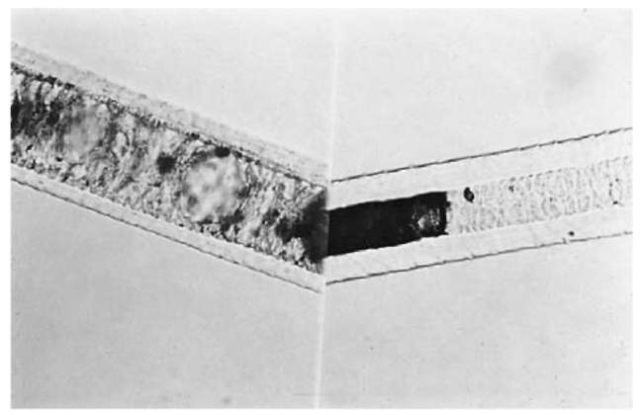
Figure 2 Left: Felidae (cat) hair; right: Canidae (dog) hair.
The following are some major identifying features of animal hairs commonly occurring in forensic casework.
Canidae: Hairs usually have a complex cellular medulla with doughnut-shaped cells (see Fig. 2). Felidae: Also have a medulla with doughnut-shaped cells (Fig. 2). Can be distinguished from canidae in that the ‘doughnuts’ are larger and the medulla shows a sudden taper at the proximal end. Hairs usually exhibit distal banding.
Cervidae: Hairs have a very broad cellular medulla with a ‘cobblestone’ effect.
Bovidae: Cortex often has large ovoid bodies. Medulla is relatively narrow and can be absent, fragmental or continuous.
Equidae: Hairs often have transverse stria, and scanty pigment irregularly distributed. Leporidae: Hairs have a multi serial ladder-type medulla and dog bone-shaped cross-sections.
As a supplement to macroscopic and microscopic animal hair identification, DNA and electrophoretic methods of species identification have recently been developed. It is anticipated that, in the future, such methods will gain widespread use in forensic laboratories.
Human Hair Identification
With human hair, the following information can usually be determined:
Body area of origin
Scalp hairs (Fig. 1) tend to be long with fairly constant diameters and cut tips. They can show evidence of treatment. Pubic hairs (Fig. 3) are generally kinky with wide variations in diameter. Their tips are frequently rounded or frayed from rubbing on clothing. Most beard hairs (Fig. 4) are broad in diameter, with triangular cross-sections. Eyebrow, eyelash and nasal hairs (Fig. 5) tend to be short and stubby, tapering to an abrupt point. Mascara or mucus may be adhering. Axillary hairs resemble pubic hairs except that they are less kinky and present a bleached appearance distally. The remaining hairs on the human body are generally indistinguishable from each other and are grouped together as general body hairs. It should be remembered that hairs on the periphery of various body regions will show combination characteristics, thereby making them difficult to identify (e.g. sideburns will show some characteristics of scalp hair and some of beard hair).
Method of removal
If a root sheath (Fig. 6) is present and/or the hair is in the anagen growth phase (Fig. 7), an examiner can conclude that the hair is indicative of forcible removal. (Forcible removal includes vigorous brushing or combing and inadvertent snagging.) A telogen root (Fig. 8), without a sheath, indicates that the hair fell out naturally. If the root is not present, an even break with regular edges indicates that it was cut off, and an irregular break generally means that the hair was broken off.
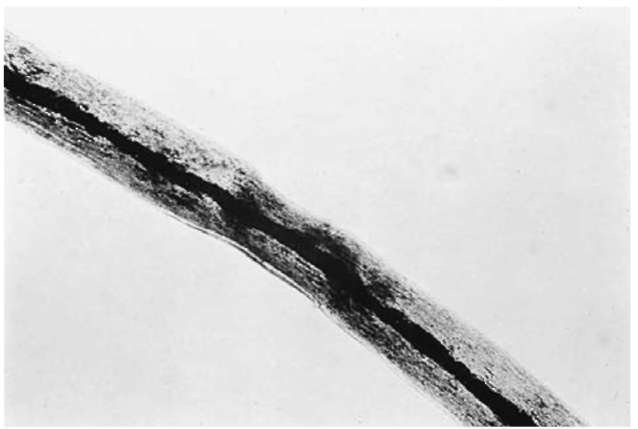
Figure 3 A human pubic hair (100 x).
Racial origin
As a result of interbreeding there are few racially pure people. Accordingly, a forensic scientist can only give an indication of the racial origin of a hair. When stating racial origin, it should be pointed out that whereas a hair may show indications of a particular race, the other features of a person may or may not. Caucasian hairs generally have variable, nonblack color; fine to medium, fairly evenly distributed pigment granules; thin to medium cuticles; and only slight variations in diameter along the shaft. Negroid hairs are generally black or dark brown with medium to coarse pigment granules; clumped pigment; medium to thick cuticles; flat cross-sectional shapes and apparent wide variations in diameter along the shaft. Mongoloid hairs are usually black or dark brown with high pigment density; coarse pigment granules; thick cuticles; round cross-sectional shapes; and constant diameter along the shaft.

Figure 4 A human beard hair (100 x).
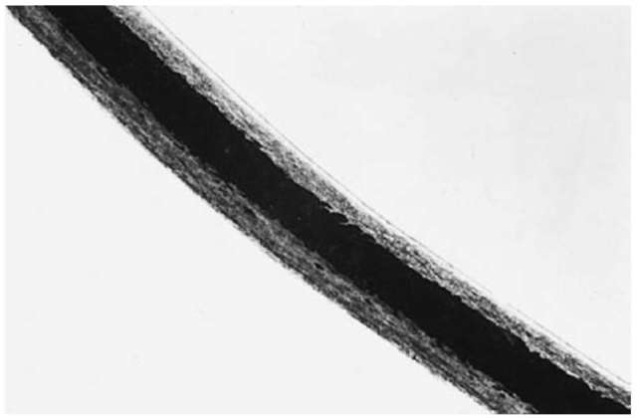
Figure 5 A human eyebrow hair (100 x).
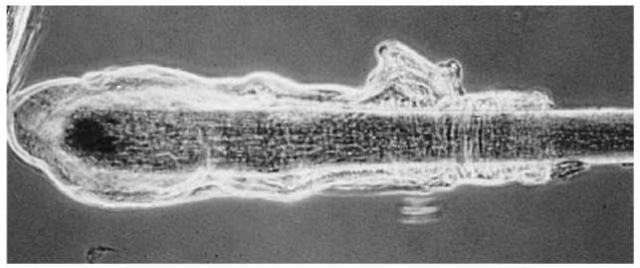
Figure 6 A root sheath attached to a human scalp hair telogen root.
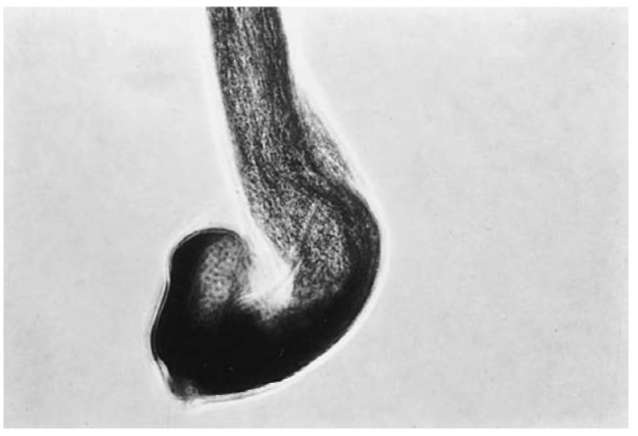
Figure 7 A human scalp hair with an anagen root.
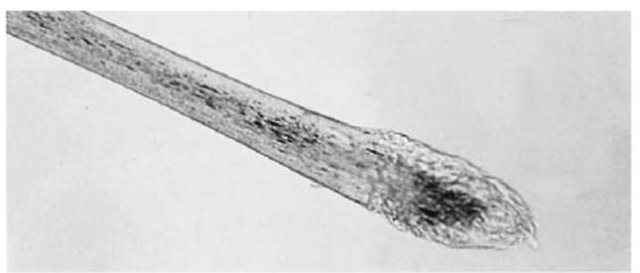
Figure 8 A human scalp hair with a telogen root.
Cosmetic treatment
Microscopical examination An abrupt color change to a very light color indicates that the hair has been bleached.
Dyed hairs also show abrupt color changes and can have unnatural microscopic colors. The presence of color in the cuticle provides another indication of dyeing. Some dyes produce very subtle color changes. If an examiner is in doubt as to whether or not a hair has been dyed, a microscopical examination with incident fluorescence illumination can be conducted. At certain excitation wavelengths, an abrupt difference in fluorescent characteristics can often be detected between the dyed area of a hair and the undyed area. Knowledge of hair growth rates combined with measurement of the untreated portion of the hair can provide an estimate of the time since bleaching or dyeing. A curly appearance accompanied by constrictions in the shaft (and often by a damaged cuticle) is indicative of permanent waving. Examination of the hair under a scanning electron microscope can sometimes reveal thickly applied hair sprays.
Methylene blue infiltration test for bleached and permanent waved hair Place a hair, cleaned by washing in water and ethanol, in a shallow dish containing a solution of 5% NH4OH in 25% by volume ethanol. Cover and allow to stand for about 10min. Then transfer the hair to a 50% ethanol solution for another 10 min. After several washings with 95% ethanol, thoroughly air dry the hair and place it in a shallow dish. Cover the hair with a 1% solution of methylene blue stain and allow to stand for 5 min. Then rinse the hair with distilled water, wash with 95% ethanol, air dry the hair and mount it on a microscope slide using a mounting medium of refractive index of about 1.52 and a cover slip.
Under the microscope, bleached hair can then be detected as a penetration of the blue stain through the cuticle into the cortex. The amount of penetration of the dye and the depth of blue color produced will be proportional to the amount of bleaching of the hair.
Permanently waved hair can also be detected with this technique. In this case, the stain penetrates, but not to the extent seen with bleached hair. Usually there is penetration past the cuticle and slightly into the cortex. Instead of deep blue, the hair appears greenish blue.
Untreated hairs will be unaffected by the stain and will appear normal. Damaged hair will often allow penetration of the dye but the hair will not be stained with any consistency.
The methylene blue can be removed from the hair by demounting the hair, removing the mounting medium from the hair by rinsing in toluene, and placing the hair in a 10% acetic acid solution heated to almost boiling for about 6 min.
Another promising procedure uses staining with Rhodamine B in combination with fluorescence microscopy. It is claimed that this procedure gives a higher percentage of correct determinations of bleached and permanently waved hairs than does methylene blue staining.
Detection of semipermanent hair dyes Semipermanent hair dyes may be detected on hair by means of thin layer chromatography as used to detect dyes on fibers.
Detection of oxidative dyes and other hair care products Gas chromatography (GC) and gas chro-matography-mass spectrometry (GC-MS) have been used to detect various hair-care products. Residues from 66 of 102 brands of hair care products (such as hair tonics, hair creams and waveset lotions) could be detected on hairs by means of GC and GC-MS analysis subsequent to ether extraction. In a later study with similar methodology, residues of four of twelve brands of hair spray and two of eleven brands of hair growth promoter were detected. Combination of the GC and GC-MS method with the thin layer chroma-tography method was found to increase discrimination in detection of oxidative hair dyes. However, identification of brand and type of hair-care products requires care because some components of oxidative hair dyes overlap with those of hair creams, waveset lotions, and hair rinses.
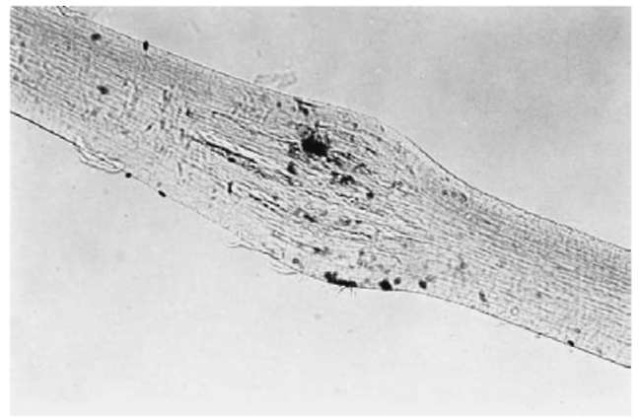
Figure 9 A crushed human scalp hair.
Damage
The shaft of a cut hair ends abruptly as opposed to the natural taper of uncut hair. If a hair has been recently cut, the margins are usually sharp and well-defined. With the passage of time, these margins become progressively more rounded off until the entire tip appears rounded.
Burned hair has a brittle charred appearance with large round vacuoles at the point of burning. Extent and type of damage depends on the temperature.
The tunneling of hair by fungal hyphae can produce distinctive transverse lines. Such damage can demonstrate that a hair has been exposed to fungi. This typically occurs with buried bodies. Another effect often seen in hair from people who have been dead for some time is termed ‘postmortem root banding’, whereby an opaque band about 0.5 mm above the root bulb can be observed with transmitted light microscopic examination.
Sex
At present, the sex of a hair donor can generally only be determined if a root sheath is present. The best methods for sex determination from hair root sheaths use forensic DNA analysis. In laboratories without access to forensic DNA analysis, the following procedure can be used.
After soaking the root and sheath in physiological saline for 3 h, immerse the hair in 25% v/v acetic acid for 1-2 min. After removing the excess acid and allowing the hair to air dry for 10 s, prepare cell ‘tracks’ by rubbing the hair bulb or root sheath in a zig-zag fashion within the circle on a specially prepared glass microscope slide. (Preparation involves inscribing a 1 cm diameter circle with a diamond pencil and cleaning the slide with alcohol.) Allow the preparation to air dry for at least 10min, then cover the area ofthe circle with a 0.5%, w/v, aqueous quinacrine dihydrochloride stain. After 3 min, wash the stain with a gentle stream of tap water and immerse the slide in a beaker of water to destain for 6 min. To differentiate the cells, add McIlvaine’s buffer at pH 5.5 (43.1 ml of 0.1 M citric acid and 56.9 ml of 0.2 M Na2HPO4), cover the preparation with a coverslip and let stand for 5 min. Read the slides at 1000x on an incident light fluorescence microscope using blue light excitation and a 100x objective equipped with an iris diaphragm. The Y-chromosome appears as a brightly fluorescing spot 0.5-1 mm in diameter. Less than 10% of the cells having such spots indicates that the hair is female; more than 50% spots indicates a male. It is advisable to confirm these determinations by an aceto-orcein staining procedure for sex chromatin in which the cells are stained with a cooled and filtered solution of 0.5 g synthetic orcein in 22 ml of hot (80-85°C) acetic acid and 27 ml distilled water. Examination is conducted at about 450x under transmitted bright field illumination. In this test, staining of sex chromatin in more than 40% of the cells indicates a female and staining of sex chromatin in less than 10% of cells indicates a male.
A variation on the fluorescence procedure has been suggested whereby the same preparation is used for both X and Y chromosomes. Staining is conducted at pH 5.5 for Y chromosomes and at pH 3.0 for X chromosomes. The Y —X score is then calculated, a score of 22 or more indicating a male and a score of less than 11 indicates a female.
Age
It is not generally possible to determine the age of an individual from which a hair originated. As an investigative aid, an experienced hair examiner can occasionally determine if a hair is from a very old or very young person. This is based on examination of the following characteristics which tend to increase with
Appendix:Characteristics for Hair Identification and Comparison
The following characteristics were developed in 1998 by a subcommittee of TWGMAT (Technical Working Group for Materials Analysis Technology) and represent an updating of the list of characteristics originally developed in the early 1980s by the Committee on Forensic Hair Comparison.
• Hue: colorless; blonde; red; light brown; medium brown; dark brown; black; other
• Pigment density: absent; light; medium; heavy; opaque
• Pigment distribution: uniform; peripheral; onesided; random; central/medial; pigment in cuticle; banded
• Pigment aggregation: streaked; clumped; patchy
• Pigment aggregate size: large; medium; small
• Pigment shape: round; oval; oblong; other
• Pigment size: large; medium; small
• Artificial treatment: dyed; bleached; other
• Length variation
• Curvature
• Form: straight; wavy; curly; twist; tightly coiled; crimp
• Diameter variation or range
• Cross-sectional shape: round; oval; triangular; flattened; other
• Other shaft configurations: buckling; convoluting; shouldering; undulating; splitting; regular; other
• Cuticular thickness: thin; medium; thick
• Outer cuticle margin: flattened/smooth; serrated; cracked; looped; other
