Introduction
The phenomenon of fire is a complex scientific event. It has had many simplified definitions applied to it in an attempt to provide a simple explanation for those who require a working knowledge of fire for practical purposes. From a forensic science viewpoint, this applies to those who are required to investigate and understand the ignition and propagation of fire, i.e. fire (cause) investigators. This knowledge must include a basic understanding of fire chemistry. This article outlines the basic chemical concepts that apply to combustion. It also defines numerous physical and chemical properties of gases and liquids that are necessary for their ignition and combustion and also explains their ‘performance’ on undergoing combustion.
A simple but scientifically accurate definition of fire (combustion) is as follows.
Fire is an exothermic, self-sustaining chemical reaction involving a solid, liquid or gas-phase fuel and it is usually associated with the oxidation of this fuelby atmospheric oxygen with the emission of energy in the form of heat and light.
It is evident then, that a fire requires fueland oxygen and produces energy and oxidation products. However, unless the fuel undergoes autoignition whereby it does not require an externalheat source, an external ignition source (piloted ignition) must be supplied before a self-sustaining exothermic chemical reaction will commence. This is the basis of the simplified ‘fire triangle’ (Fig. 1) that appears in all fire investigation texts and, when the need for a chemicalchain reaction is included, leads to the more complicated ‘fire tetrahedron’ model(Fig. 2). If a side of the ‘fire triangle’ or a face of the ‘fire tetrahedron’ are not present then combustion can not occur. Simply, the physical requirements for fire are outlined below.
Fire
Conditions for fire
Air – Oxygen As mentioned in the definition of fire, the oxidizing agent is usually atmospheric oxygen. As will be explained later, the actual presence of oxygen is usually easily explained by the fact that it constitutes approximately 20% of the atmosphere. However, the amount of oxygen available is a critical parameter in the spread and intensity of a fire.
Under favorable conditions, oxygen can be supplied to a fire by the presence of other oxidizing agents, for example, potassium chlorate (KQO3) and sodium nitrate (NaNOs), which contain oxygen in their chemicalstructure. Also, under rare conditions, combustion can occur in an atmosphere of carbon dioxide or other gas in the absence of oxygen.
Fuel From a practicalviewpoint, the fuel referred to in the definition can be regarded as any materialthat exists in a chemicalstate in which it can be oxidized by oxygen in the presence of a suitable ignition source.
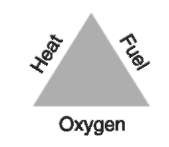
Figure 1 The fire triangle.
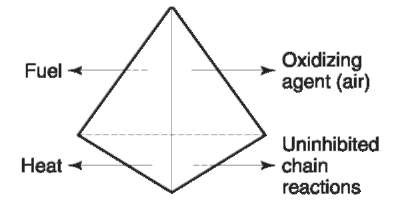
Figure 2 The fire tetrahedron.
The common fuels requiring consideration in fire investigation are organic compounds usually containing significant amounts of carbon (approx. 50% and higher) and hydrogen. They include naturally occurring compounds such as wood, cotton, etc., synthetic materials such as plastics, paints, rubbers, etc. and refined fuels and solvents which include petrol, lighting kerosene and methylated spirits.
Heat Energy in the form of heat is necessary to excite the molecules of both the fuel and the oxygen to an activated state required for chemicalreaction. The minimum temperature necessary to initiate the ‘self-sustaining chemical reaction’ referred to in the definition of fire, is known as the ignition temperature of the fuel. It is the source of ignition that is the primary interest of fire investigators because this can be interpreted as the cause of the fire.
Fire requirements and processes
When a fueland an oxidizing agent are heated to ignition temperature, either through autoignition or piloted ignition, chemical reactions occur. These exothermic (heat-producing) reactions provide additional energy to the situation and, as a consequence, further reactions proceed. When the production of excited species exceeds the decay of these species, the chemi-calreactions can be regarded as a chain or self-sustaining reaction and a fire or explosion results. The mechanism for this process is further discussed under ‘Flame Chemistry’ below.
The fire will continue until one of the reactants or the heat providing the energy source for continued ignition is expended. This forms the basis of methods for the containment and extinguishment of fire. Examples of fire-fighting principles include removing the fuelload, removing the oxygen source by covering a fire in foam or an inert gas and removing the heat supply by hosing with water on the principle that the latent heat of vaporization of water accounts for the heat.
Although combustible organic compounds exist in the solid, liquid or gaseous phase, most of these fuels will only burn when in the vapor phase, that is, a combustible gas-phase mixture must exist. For many volatile liquids this gas-phase mixture exists at ambient temperatures, but for others the liquid must be heated to a temperature at which the liquid will exert a vapor pressure which, when mixed with the required amount of oxygen, can support combustion. This is known as the flash point of the fuel. Simply, the flash point is the lowest temperature at which a liquid produces a flammable vapor. This is not to say, however, that the liquid will ignite if heated to this temperature; it will still require an ignition source at the required ignition temperature. Furthermore, the flash point temperature will not sustain a fire. In practice, combustion requires a slightly higher temperature known as the flame point, to achieve this.
Although the flash point determines the minimum temperature at which a gas-air mixture meets the requirements for ignition, there is also a maximum temperature, above which, a liquid will exert a vapor pressure that is so high, that the required amount of air or oxygen will not be available for ignition. Therefore, there exists both an upper and lower flash point for liquid fuels. Most fire investigation texts report only the lower flash point because, in an actual fire situation, uncontrolled ventilation renders the upper flash point of academic interest only.
The preceding discussion refers to the need to evaporate a liquid, that is, to convert the liquid into the gas phase. A phase change from solid to liquid or liquid to gas requires an energy input referred to as latent heat. In the latter case, this is known as the latent heat of vaporization and is defined as the quantity of heat absorbed by a substance in passing between liquid and gaseous phases. A flammable liquid with a high latent heat of vaporization will require a high heat input to convert it into the combustible gas phase and, as a consequence, evaporation rates are limited and the heat is unavailable for other combustion processes such as pyrolysis. An example of a substance with a relatively high latent heat of vaporization is ethanol. Most are familiar with the cold sensation when methylated spirits comes in contact with the skin. This is due to the absorption of body heat in the vaporization process. The latent heat of vaporization is, therefore, another in a series of chemicaland physicalproperties of liquids that determine their effectiveness as an agents to assist the ignition and spread of fire, i.e. to act as an accelerant.
Solid organic fuels, including natural products, such as wood, and synthetic products, such as plastics and rubbers, have a chemicalstructure that is based on high-molecular-weight molecules. In the case of wood, these materials include lignins and the naturally occurring polymeric carbohydrate cellulose, whereas in the case of plastics, synthetic polymers based on monomers, such as styrene and vinyl, are present. If these materials are to burn, they must first be thermally degraded by a process known as pyro-lysis, to small molecules that exist in the gas phase.
Pyrolysis occurs when sufficient energy is applied to break chemicalbonds, thus reducing large molecules to low-molecular-weight compounds. In many cases, these smaller compounds include monomers and dimers that were used to construct the polymeric compounds undergoing thermaldecomposition.
These pyrolysis products should not be confused with free radicals (see ‘Flame Chemistry’) that are also produced during thermal decomposition, but they will themselves become a source of free radicals.
The processes of evaporation and pyrolysis explain the generalobservation that flames occur above the surface of a solid undergoing flaming combustion rather than at the surface. There are important exceptions to this process and these occur when carbon (char) and some metals, e.g. aluminum and magnesium, burn. In these cases, there is direct oxidation at the surface of the solid and the process is normally very efficient, that is, the products of combustion can not be further oxidized. In the case of burning carbon, the process is referred to as glowing and, when complete combustion occurs, the combustion product is carbon dioxide. However, if the oxygen supply is restricted, incomplete combustion may occur with the production of carbon monoxide; a gas which is often responsible for deaths in fires. The subsequent combustion of this gas to carbon dioxide is characterized by a blue flame above the ‘glowing’ solid.
If combustion (i.e. oxidation) is 100% efficient, the following chemical equation applies for hydrocarbons (compounds containing carbon and hydrogen only) regardless of their origin:

For toluene, a major component of petroleum, the equation is
![]()
However, for uncontrolled combustion, where there is limited or no control over the supply of combustion reactants and removalof combustion products, the following equation more accurately defines the situation.
Hydrocarbon + Oxygen + heat — Carbon Dioxide + Water + Heat + products of incomplete combustion
(carbon monoxide, soot, pyrolysis products, polynuc-lear aromatic hydrocarbons, etc.)
Again, for toluene, with a limited supply of air, the equation could be

It is the products of incomplete combustion that form the smoke at the scene of a fire. Smoke is the cloud of soot particles, particulate matter, unburnt gases and combustion gases which, as a result of convection, rises above the fire. However, even with efficient combustion there may be gray ‘smoke’ seen in the gas plume due to condensing water vapor. The products of incomplete combustion may themselves be subsequently oxidized in a poorly ventilated fire. If such a fire is suddenly ventilated, these products may reach their explosive limits (see later) and ignite explosively in a process known as ‘flashback’ or ‘smoke explosion’.
Flame Chemistry
Fundamentally, the investigation of fire origins and causes requires an understanding of fire-travelin-dicators such as depth of burning, lowest point of burning, etc. ‘Glowing’ fires, although extremely efficient oxidation processes, do not contribute significantly to fire spread, particularly early in the development and spread of fires and are usually associated with the middle and final stages. Most of the effect of fire can be attributed to the convection and radiation of heat from flames and, as such, an understanding of the basic chemistry of flames, which are the source of that heat, is where the science of fire investigation actually commences.
The various physicalproperties and parameters of gaseous fuels, the formation of combustible gases from liquids and solids and the requirements for ignition and combustion have been discussed. Also, the formation of oxidized and semioxidized products of combustion have been briefly reviewed. It remains to consider how this oxidation actually occurs in flames and why energy in the form of heat and light is produced.
Basically, the energy contained in any combustible materialis a direct consequence of the chemical bonds that bind the material together. It is the breaking of these chemical bonds that releases this energy and oxidation is one chemical process or reaction that will achieve this. The amount of energy released in this way, i.e. by means of an exothermic chemical reaction, depends on the chemical composition of the reactants and the chemical composition of the products. (This is not to say that all chemical reactions are exothermic. On the contrary, many chemical reactions are endothermic and require the input of energy to allow the reaction to proceed and form products which have chemicalbonds of higher chemicalenergy than the reaction products.) This release of energy occurs within the flame zone and, although flame structures and compositions are extremely complex, energy released can be explained simply as a consequence of the formation and properties of free radicals.
Free radicals are formed through the breaking of molecular bonds and are described as molecular fragments possessing one or more unpaired electrons. Because unpaired electrons are extremely susceptible to pairing with other unpaired electrons to form chemical bonds, these species are very reactive and short-lived. Any combination of free radicals is associated with the evolution of energy as the species combine to form a more stable entity.
During a fire, gaseous fuel (that could also be present due to an evaporated liquid or a pyrolyzed solid) forms a region rich in fuelvapor. Where this region comes in contact with an oxidant-rich region the gas phase combustion zone forms which is characterized by a thin flame sheet. Here the fuel is dissociated into extremely reactive free radicals that immediately react with atmospheric oxygen (and nitrogen) to form the finalcombustion products which, depending on fuel and oxidant conditions, are at various levels of oxidation. The energy released by these oxidation processes is radiated in all directions, including back to the fuelsource and this insures that the chain reaction continues and the flaming area spreads. For small steady flames characterized by burning candles, cigarette lighters, etc., these flames are known as laminar diffusion flames. For larger unstable fires, they are known as turbulent diffusion flames.
There are some important consequences of free radicalformation for fire suppression and fire investigation.
1. The removal of these free radicals provides an additionalmethod of fire suppression and this has been utilized by fighting fires with chemical agents and chlorinated hydrocarbons.
2. The amount of energy released by fuels can be calculated. This can provide valuable explanations for the speed of fire spread and/or the intensity of damage. This value is known as the heat of combustion or heat output of the fuel. A simple definition for the heat of combustion is the maximum amount of heat that can be released by the complete combustion of a unit mass of combustible material.
This value can then be used to calculate a theoretical adiabatic (no heat gain or loss from the system) flame temperature that can provide information concerning the effect a flame can have on materials in the immediate area. There is, however, a further consideration before fire-spread can be predicted. This is the specific heat of fuels in the immediate area. The specific heat is the amount of thermalenergy required to raise a unit mass of a substance 1°C.
Because fire is dependent on fuels attaining ignition temperature, the capacity of a fuel to absorb heat is yet another criticalparameter in a consideration of fire spread. It effectively indicates the potential of a fuel to reach a dangerous condition or state during exposure to a fire and it is therefore an important consideration in fire suppression and fire-pattern interpretation. The converse property, i.e. the resistance of a material to absorb heat, is known as the thermal inertia of the substance. Specific heat varies over a considerable range for common fuels but is less than 1 calg”1 °C-\
Another important consequence of exothermic reactions is that, for an increase in ambient temperature, there will be an increase in the reaction rate. This gives rise to the concept of a Q10 value for chemical reactions. The Q10 value is defined as the increase in the rate of a chemicalreaction that results from a temperature increase of 10°C. Generally, the Q10 value exceeds 2, which effectively means that, for every rise of 10°C the rate of the reaction doubles. Although this value assists in explaining why fires appear to grow at a rate that exceeds a simple time-development relationship, it would suggest that fires should rapidly become a conflagration. The fact that this does not happen is due to the limits on fuel availability and the increasing heat loss to the surroundings.
As previously stated, flames are primarily responsible for fire development and spread. Under uncontrolled conditions, flame combustion is a less efficient oxidation process than combustion which results from ‘glowing’. This is easily confirmed by observations which revealthat a range of partially oxidized combustion products, such as soot, pyrolysis products and charcoalformation, are present with flaming combustion but are not seen during ‘glowing’. In fact, the simple presence of a yellow flame often indicates that incomplete combustion is occurring because the flame color is due to the presence of incandescent carbon particles.
Explosions
The mechanisms of explosions are examined in detail in other sections in this text. However, because they represent a particular form of combustion they require some consideration here.
An explosion is effectively a rapid increase in pressure due to the formation of gases during combustion or detonation and/or the rapid heating of atmospheric gases. If this is to occur in the burning of hydrocarbons in the gas phase, certain conditions must be met.
Fuel-air (diffuse) explosions
In fires, the burning rate is controlled by the amount of oxygen available, that is the ventilation of the fire, and the fuel available for combustion. The supply of fuel is itself dependent on the rate of burning and therefore on the rate of pyrolysis. Therefore, to a certain extent, the production of flammable gases and their mixing with air is dependent on the fire itself. In an uncon-fined area and while there is adequate ventilation,products of combustion are removed from the system, air enters the system and the combustion process continues. However, in the case of explosions, the air and vaporized fuelare intimately mixed before ignition and, as a result, the ignition is extremely rapid. If this rapid combustion occurs in a confined space, then the resulting products of combustion and the existing atmospheric gases undergo a large and rapid expansion and the effects of an explosion are experienced. The pressure wave resulting from this rapid combustion is in the order of approximately a 1500 kgm”2.
Explosions are commonly associated with hydrocarbon vapors, but serious incidents also arise with other fuels in the form of vapors, mists, foams and dust suspensions. All these materials contribute to a type of explosion known as a diffuse explosion that is characterized by the presence of a combustible fuel-air mix in which the air is the oxidizing agent.
It was mentioned above that upper and lower flashpoints determine the temperatures at which vapors of liquid fuels can be ignited. It follows, therefore, that there must be particular concentrations of vapor and air which will support and sustain combustion. These concentrations are known as the explosive limits of the fuel. Simply stated, the explosive limits of a fuel are the upper and lower concentration limits of vapor in air which will allow explosive combustion. Because vapor pressures of combustible liquids decrease with increases in envi-ronmentalor ambient pressure, flash points must increase with increasing pressure as a consequence.
There are, however, instances when explosions will occur although the fuel vapor-air mixtures do not fall within the upper and lower limits. If a fuel vapor-air mixture is too lean (insufficient fuel vapor concentration) and an ignition source is applied, this can result in localized heating which raises the localized fuel vapor pressure to a value within the explosive limits and an explosion will occur. Conversely, if a fuel vapor-air mixture is too rich (insufficient air concentration), the application of a heat source might cause enough turbulence to produce a localized fuel vapor-air mixture to fall within the explosive limits. Again an explosion can result.
For hydrocarbon fuels in a practical situation, the lower or lean limit often leads to an explosion only, with considerable disruption whereas the upper, or rich, limit is usually associated with an explosion and a significant fire. It is unlikely that the fuel vapor-air mixture would be consistent throughout an entire structure. It is more than likely that there are zones where the mixture is too lean to explode and other areas where it is too rich. It is equally likely that a zone exists between these extremes where the mixture meets the requirements for an explosion. Should an ignition source be present in this area, a ‘rolling’ fire or explosion can result.
Technically, in the case of explosive combustion, there are three alternative processes. If the mixture of fuel and oxidant (generally, air) is homogeneous and the conditions for combustion are met, the chemical reactions occur throughout the entire reaction mass. This is known as a uniform reaction. Alternatively, if there is a clearly defined reaction zone (flame) separating zones containing predominantly fuel and zones containing predominantly oxidant, then the reaction zone (flame) will move through the reaction mass. This is known as a propagating reaction. In this case, the propagation velocity at which the reaction zone or flame moves, further defines this type of combustion. When the reaction zone velocity is less than the velocity of sound (i.e. subsonic) the reaction is known as a deflagration and when it exceeds the velocity of sound (supersonic) it is termed a detonation.
Dense-phase (concentrated) explosions
Dense-phase or concentrated explosions differ significantly in that they are not dependent on oxygen in the air but result from an oxidation made possible because the explosive compounds contain both the fuel and the oxidant. With the input of a small energy source, the chemicalstructure of these compounds is rearranged with the simultaneous production of much more energy. This energy can be measured and is known as the heat of decomposition. Common explosives in this class include dynamite, trinitrotoluene (TNT), pentaerythritoltetranitrate (PETN) and nitroglycerine. More details concerning the structure of explosives and the mechanisms of explosions are provided in other articles.
In most actualfire scene situations, many of the principles and processes mentioned always apply. However, the relative significance of many of these will vary with the actual situation and relatively insignificant changes in one or more of the fire conditions may have a profound effect on other processes and effects.
