Introduction
During the past 50 years in North America, breath has emerged as the biological specimen of choice for the forensic analysis of alcohol (‘alcohol’ will refer to ethyl alcohol) in routine drunk driving cases. Amid some controversy, forensic breath alcohol analysis has only recently become more widespread in Europe where blood and urine alcohol programs have long-been established. Despite many legal challenges regarding perceived deficiencies in breath alcohol measurement, expanding forensic application continues. There are many reasons for preferring breath over other biological specimens including: (1) less invasive sampling, (2) rapid analysis and reporting of results, (3) expanding legal support and acceptance, (4) impairment research relying predominantly on breath alcohol data, (5) minimal training of operators, (6) easily adapted to field testing environments and (7) health risks associated with the collection and preservation of biological fluids. Forensic breath alcohol measurement, therefore, merits special consideration and discussion.
This article presents the historical, biological, analytical, administrative, legal and future considerations of forensic breath alcohol analysis. Similar to other measurements in forensic toxicology, forensic breath alcohol analysis is concerned with the measurement and interpretation of alcohol collected from a human biological system and applied within a legal context generally defining its acceptability and interpretation. This unavoidable blending of science and law adds a unique dimension and challenge to the forensic scientist who must measure and interpret breath alcohol results.
Historical Perspective
The seminal work of E.M.P. Widmark in Sweden during the 1920s regarding human alcohol physiology and analytical measurement led to widespread development of blood alcohol programs in Europe and North America. The published work of Liljes-trand and Linde in 1930 cohesively established much of the physiological theory underlying breath alcohol measurement, prompting further investigation and instrumental development. Practical methods for measuring breath alcohol were later enhanced during the 1930s when Harger developed an instrument named the Drunkometer. Jones provides an excellent overview for the historical development of all aspects of forensic alcohol analysis.
The earlier development of blood alcohol methodology resulted in the unfortunate notion that breath alcohol would be a surrogate technique and interpreted as a blood alcohol equivalent. Much debate (even to this day) has occurred in both the scientific and legal communities regarding blood and breath alcohol comparisons. Numerous legal challenges and a myriad of diverse court opinions have resulted. In an effort to remedy the confusion, legislation in both North America and Europe has been enacted which prohibits specific alcohol concentrations for both breath and blood separately. These legal measures have appropriately distinguished breath as a separate and reliably measured biological specimen worthy of forensic application and interpretation.
Biological Principles
Following the typical route of oral ingestion, alcohol will distribute itself by simple diffusion throughout all of the body water. Blood will transport alcohol through the pulmonary circulation to the lungs where partitioning by simple diffusion occurs with the associated alveolar and bronchial air as governed by Henry’s Law. The blood/air partition coefficient (Ost-wald coefficient) is approximately Xba-1750 at 37°C. Relative to other respiratory gases, alcohol will by far be the most abundant when consumed to forensically significant concentrations. Moreover, various pathological conditions will not generally preclude the accurate measurement of breath alcohol concentrations. The predictable physiological characteristics of alcohol in breath have facilitated its ability to be reliably sampled and quantified following a sustained exhalation.
Proper sampling is a critical consideration in forensic breath alcohol measurement that contributes significantly to its interpretation. Breath is highly heterogenous with regard to alcohol due largely to its high solubility in body fluids. Because of significant airway interaction on exhalation, samples will generally have lower alcohol concentrations compared to that of the alveoli. Fig.1 illustrates continuous time sampling curves for both a human and simulator sample source. Simulator devices containing known concentrations of alcohol and water at controlled temperatures are used to provide vapor alcohol samples for instrument calibration and testing purposes. Greater variability is clearly observed in the human breath sample. Moreover, any unusual pre-exhalation breathing patterns (e.g. hyper- or hypoventilation) will result in even greater sampling variability. This sampling (biological) component of breath alcohol measurement generally contributes over 80% of the total method variability as shown by summing independent variances. Since a breath sample must be obtained from a conscious and cooperative individual (often difficult when intoxicated), the combination of instrumental sampling features along with careful interaction between operator and subject is critical to obtain consistent and properly interpretable samples that merit forensic application. As with measurement in respiratory spirometry, care and attention is necessary for adequate sampling.
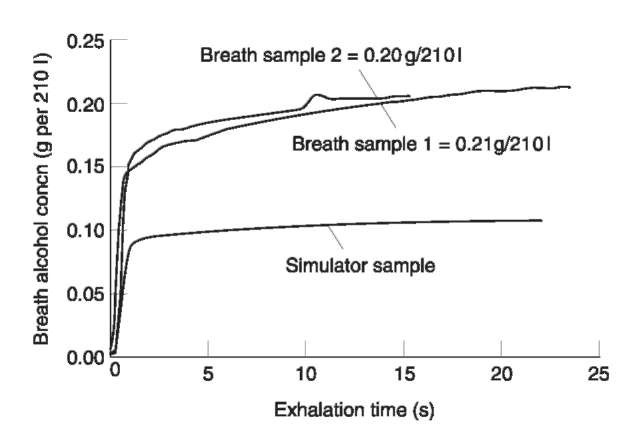
Figure 1 Continuous time exhalation profiles from a human subject and a simulator device.
Analytical Methods
Many recent advances in analytical technology have found relevant applications in breath alcohol instrumentation. Most of the commercially available instruments today employ one of the following technologies.
1. Wet chemistry: alcohol in the breath sample is oxidized in a solution containing an oxidizing reagent (e.g. potassium dichromate) resulting in a color change measured optically in accordance with Beer’s Law.
2. Infrared: alcohol in the breath sample is quantified by absorbing infrared energy filtered to specified wavelengths in accordance with Beer’s Law.
3. Electrochemical: alcohol in the breath sample is oxidized on a chemically active surface yielding electrons and resulting in a measurable increase of electrical conductance.
4. Gas chromatography: alcohol in the breath sample is passed through a treated column resulting in separation and eventual quantitation by, for example, a flame-ionization detector.
5. Dual technology: two technologies might be employed to improve the specificity for ethyl alcohol in breath samples. Infrared and electrochemical methods, for example, might both be used for quantitation and identification.
While employing one or more of the above methodologies, manufacturers add several features to enhance the forensic uniqueness of their instruments. In an effort to obtain deep lung end-expiratory samples, hardware and software features incorporate various parameters including: minimum flow rate, minimum volume, minimum exhalation time, alcohol curve slope determination, etc. Sample chamber purging and signal zeroing features are added to ensure reference levels for subsequent measurements. Various internal standards are added to ensure the proper function of optical, hardware and software features. Some infrared instruments employ filters for two or more wavelengths while attempting to improve specificity for ethyl alcohol. Automatic sampling and analysis of external standards (e.g. simulator or gas standard devices) are also added features to most modern computerized instruments. Various optical pathlengths and signal processing features are also added to improve signal-to-noise ratios, accuracy and precision. Computerization has probably advanced modern instruments the most while greatly enhancing their analytical and data collection capabilities. Table 1 lists several commonly used breath alcohol instruments in North America and Europe along with their analytical method. This list is by no means exhaustive. Generally, manufacturers are willing to customize their instruments to accommodate the unique needs of local jurisdictions.
Screening and evidential purposes
Analytical methods also vary depending on whether the intended purpose is screening or evidential. The notion of screening or evidential is largely determined by relevant rules and regulations in each jurisdiction. The same device may be employed for screening in one jurisdiction and evidential in another. Screening devices are frequently handheld and employ electrochemical technology. They are generally more operator dependent and may lack some features (e.g. sampling control, external and internal standards, etc.) because of their limited purpose. Their advantage is portability, ease of operation, and rapid analysis. Results from evidential instruments are generally used in court to prove elements of an alleged criminal offense and thereby designed to address various forensic issues likely to arise. Evidential instruments will generally be computer controlled with features including external and internal standards, sampling control, specific protocol control, automatic purging and zeroing, data collection, error detection, quality control features and printout results. Assistance for local jurisdictions in the selection and use of breath alcohol equipment is provided by national organizations in the United States (National Highway Traffic Safety Administration), in Canada (Alcohol Test Committee of the Canadian Society of Forensic Sciences) and in Europe (International Organization of Legal Metrology, OIML). These organizations have developed recommended analytical standards along with approved product lists.
Table 1 Several of the most commonly employed forensic breath alcohol instruments along with their analytical method
| Instrument | Analytical method |
| Alco-Analyzer 2100 | Gas chromatography |
| Alcolmeter | Fuel cell |
| Alcomonitor CC | Fuel cell |
| Alco-Sensor IV | Fuel cell |
| Alcotest 7110 | Infrared/fuel cell |
| Breathalyzer | Wet chemistry |
| BAC Datamaster | Infrared |
| Camec | Infrared |
| Intoximeter ECIR | Infrared/fuel cell |
| Intoxilyzer 5000 | Infrared |
Reporting results
Although different instruments offer a variety of display and printing formats, local administrative rules will generally govern those employed. Although some screening devices have a colored light system to report results, evidential instruments generally display and print results with their units truncated to two or three decimal places. Printout documents received from the instrument at the time of analysis are generally the best legal record for court presentation, avoiding further risk of error associated with transcribing, data transmission, processing and subsequent printing. The format and information content of printout documents require careful consideration because of their persuasive influence in court. Although determined largely by local regulations and issues, the information on printout documents should include: date, instrument serial number, operator name, subject name, subject date of birth, simulator standard batch number, simulator temperature verification, presampling observation time period, blank test results, breath test results, simulator standard results, associated times and units for all results.
Interpreting results
Similar to other considerations, the interpretation of results often depends on local administrative rules. Although most jurisdictions directly report the breath alcohol concentration (e.g. grams per 210 liters), some remain who must convert the value to a blood alcohol equivalent according to some assumed conversion factor. Appropriate conversion factors have generally been determined experimentally by comparing simultaneously collected blood and breath samples as illustrated in Fig.2. The limitations of this approach due to analytical and biological variability should be appreciated. Although a large body of paired blood and breath alcohol data exists showing reasonable agreement, this conversion practice is forensically unsound. Additional uncertainty and unnecessary debate are introduced with this indirect approach to measurement interpretation.
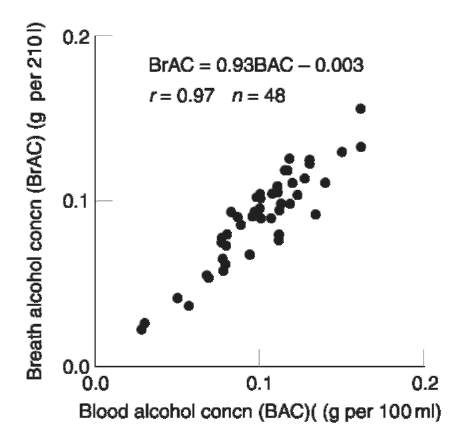
Figure 2 Scatterplot along with linear regression equation comparing essentially simultaneously collected within-subject blood and breath alcohol samples.
Computerized instrumentation
Computerized breath alcohol instrumentation offers several advantages. The measurement process can be monitored and controlled to insure conformance with quality control standards. Algorithms controlling breath sampling, signal processing, measurement protocol, error detection, reporting format, etc. can also be incorporated. In addition, data collection via modem and host computer can be accomplished. Subsequent data analysis can provide useful insight for program evaluation along with demographic and law enforcement features of drunk-driving enforcement.
Forensic considerations
Many legal challenges regarding breath alcohol have prompted manufacturers to incorporate hardware and software features addressing these matters. Minimum breath sampling parameters, for example, improve the precision among replicate breath alcohol analyses. Protocols requiring replicate breath samples along with internal and external standard and blank analyses are encoded in software requiring the operator to follow the specified test sequence. Hardware and software features allow the instrument to monitor for interfering volatile organic compounds or radio frequency interference (RFI) with the subsequent aborting of a test when detected. Other error detection capabilities are also included that monitor various instrument and test criteria. Basic design features including separation of circuitry, power line filtering, breath tube heating, continuous conductivity metal casing, stable power supply and cooled infrared detectors also minimize noise and potential interference. Dual technology and/or multiple infrared filters are often incorporated to improve specificity for ethyl alcohol. Potential interference from the multitude of endogenous volatile organic compounds is generally mitigated because of the trace levels (well below levels of detection) of these materials in normal human breath. Protocol details such as precluding the subject from observing test results until printed can also be added to minimize bias. Instruments can even be programmed to compute and report confidence intervals for mean breath alcohol results. Modern computerized instrumentation has generally enhanced the integrity of breath alcohol measurement by addressing many forensic concerns.
Analytical Protocols
Forensic breath alcohol results should be viewed as the product of an integrated measurement system including hardware, software, standards, operators, protocol, training, etc. Fig.3 illustrates various components of this total measurement system which all contribute to measurement reliability. Moreover, the forensic integrity and interpretation of results depends equally on competent instrumentation and testing protocol. Often, jurisdictions allocate significant time and resources in selecting the best possible instrument for their purposes but fail to give equal attention to their evidential testing protocols. Fig.4 illustrates the balanced consideration of both instrumentation and protocols to optimize forensic integrity and quality control.
The forensic context must be thoroughly appreciated to properly develop a breath-testing protocol that is fit-for-purpose. Other clinical measurement contexts are often inadequate models for forensic purposes since program details and interpretation of results are rarely challenged in a legal context. The profound implications of breath alcohol results should motivate a sound analytical protocol for each subject tested including: replicate breath sample analyses, replicate agreement criteria determined as a function of concentration, simulator or gas standard control measurements, blank tests, internal standard tests, presampling observation time period, instrumental and procedural error detection features and printout results. Moreover, the forthright acknowledgement when individual test results or procedures are inadequate and the full disclosure of all testing results and program policies are critical to forensic integrity. Significant legal challenge can, in some cases, motivate necessary change and improve overall quality control. Generally, forensic breath alcohol programs develop in correspondence to the intensity of legal challenge. Forensic officials would be wise to listen to the voice of their critics as well as their advocates since even the best of programs can be improved.

Figure 4 Forensic measurement quality control and confidence is a balance between both adequate instrumentation and appropriate measurement protocol.
The instrumental and protocol features necessary to achieve an acceptable level of quality control is largely determined within each jurisdiction depending on: statutory language, administrative rules, legal case law, common defense challenges, political and administrative considerations, training requirements, program costs and funding sources, etc. Several features that will generally enhance forensic integrity include: carefully drafted administrative rules, formally approved and tested instrumentation, formally trained and approved personnel, formally approved training outlines, formally approved instrument software versions, formally approved test protocol and standards, etc. These elements comprise the ‘information matrix’ available to the forensic scientist who interprets and communicates test results in court. The critically important interpretation and clear communication of breath alcohol results is the final stage of the total measurement process. Without careful consideration and appreciation, more ‘noise’ may be introduced into breath alcohol results at this final stage of communication than during all previous stages of sample collection and analysis.
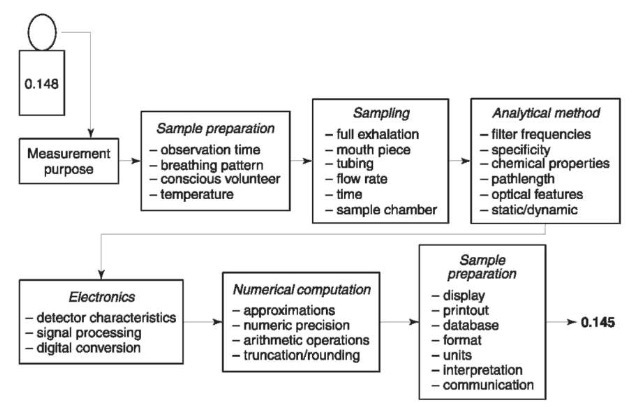
Figure 3 Breath alcohol measurement algorithm showing critical elements that comprise the total measurement system.
Program Administration
Forensic breath alcohol programs in North America can be administered in part or in whole at either local, state or federal levels. European programs, on the other hand, are generally administered at the federal level. Administrative rules defining program structure and policy have varying degrees of detail. These rules must be carefully constructed in view of the law and case law history since they generally define the legal admissibility and are often the focus of legal challenge. Technical detail should be minimized. Development of adequate administrative rules requires the collaborative effort of law enforcement, scientific personnel, prosecuting authorities, defense interests, court personnel, funding agencies, etc.
Two important agencies in the United States that have assisted jurisdictions in program development and administration include the National Highway Traffic Safety Administration (NHTSA) and The National Safety Council Committee on Alcohol and Other Drugs (CAOD). NHTSA has developed instrument testing protocols as well as an approved products list identifying instruments complying with published standards. The CAOD has published guidelines, policy statements and recommendations regarding many aspects of forensic breath alcohol programs.
The increased application of data collection systems has resulted from the capabilities of modern computerized instruments. Many interested agencies find applications for these data to evaluate program features including: law enforcement effort, instrument performance, demographics of arrested subjects, trends in program performance, legislative issues, defense challenges and court admissibility and interpretation. The current intrigue with computer technology is likely to increase the demand for forensic breath alcohol data collection and analysis.
Since most forensic breath-testing programs are operated by governmental agencies, program costs are an important consideration when competing for limited resources. The costs of instrumentation, personnel, training, maintenance and protocol features must all be balanced against quality control and overall forensic objectives. Many jurisdictions have cleverly supplemented their funding with penalty fees collected from the convicted drunk driver.
Legal Context
The relevance and analytical detail of forensic breath alcohol measurement is more fully appreciated within the broader adversarial legal context. To a large extent, breath alcohol testing programs, including instrumental and protocol features, are the product of continuing adversarial challenge. Program administrators often find it easier to address challenges by changing some feature of the instrument or protocol rather than continually debating the merits of the challenge. The forensic context adds a dynamic element continually challenging program structure and policy.
Typical statutory language
The trend in recent years has been to express the drunk-driving offense as prohibiting separate specified blood and breath alcohol concentrations. These ‘per se’ expressions of separately prohibited concentrations have been attempts to simplify the analytical interpretation and avoid unwarranted and time consuming argument. Per se language, however, has not been without difficulty. A more focused challenge on all aspects of the breath alcohol test has resulted. A per se law provides little else to challenge.
The scientific and legal justification for these statutory measures has been due, in part, to several large epidemiological studies revealing breath alcohol as a suitable surrogate measure for driving impairment. The Uniform Vehicle Code illustrates statutory language closely followed by many jurisdictions in the United States: ‘A person shall not drive or be in actual physical control of any vehicle while: 1. The alcohol concentration in such person’s blood or breath is 0.08 or more based on the definition of blood and breath units in §11-903(a) (5)’. Moreover, many jurisdictions define different offenses and allow varying penalties at different breath alcohol concentrations. Implied Consent statutes are an important adjunct allowing law enforcement personnel to obtain breath samples more readily from arrested subjects. Federal funding in the United States has also been an important motivation for states to enact specified legislation in several drunk-driving related areas. This increased legal and public attention on breath alcohol analysis through more complex laws, enhanced penalties, and funding enticements are important considerations for program development and forensic application.
Case law history
Compared to other nations, the United States (with its litigious propensity) probably has the largest body of case law regarding forensic breath alcohol analysis. The case law in each jurisdiction will generally be unique because of statutory language and case law history. Major federal cases including US v. Frye (1925) and Daubert v. Merrel Dow (1993) have influenced constitutional issues regarding the scientific acceptability and legal admissibility of breath alcohol results. The case law history is an important consideration influencing program development, forensic admissibility and interpretation in specific legal contexts.
Common legal challenges and responses
The serious consequences of a drunk-driving conviction have evoked vigorous and exhaustive defense challenge to forensic breath alcohol analysis. Although some challenges are unique to specific jurisdictions, many are more universal. Several of these legal challenges along with typical prosecution responses include the following.
1. Biological issues: the individual may have an elevated body temperature, deficient respiratory capacity, abnormal breathing pattern, abnormal hematocrit, ‘mouth alcohol’, etc. The typical response is to argue that the law does not distinguish between these issues, therefore, they are not a defense. These matters should go to the weight of the evidence and not their admissibility. Some biological issues (e.g. abnormal breathing pattern and ‘mouth alcohol’) are controlled by instrumental and protocol considerations.
2. Interfering substances: the individual may have volatile organic compounds in their breath from either endogenous or exogenous sources. Research evidence tends to minimize the potential for bias resulting from exposure to these substances when proper forensic protocols are followed. Instruments are designed, generally, to preclude bias from specific interfering substances.
3. Results near ‘per se’ level: results just exceeding per se levels may be in error with the possibility that the ‘true’ value is below. The uncertainty in breath alcohol measurement must be acknowledged. Appropriate testing protocols can allow for estimates of systematic and random error and determination of confidence intervals.
4. Measurement performed subsequent to time of driving: Results at the time of analysis are argued to be higher than at the time of driving where laws prohibit specified breath alcohol levels ‘at the time of driving’. Many jurisdictions have enacted laws prohibiting specific breath alcohol levels within some specified time of driving (e.g. within two hours). Moreover, experimental evidence suggests that analytical results subsequent to driving will be equal or less.
5. Alcohol in the breath does not produce impairment: breath alcohol has long been a recognized index of impairment with most court appeals upholding the constitutionality of breath-specific per se laws.
6. Analytical deficiencies: alleged deficiencies in instrument performance have somehow biased the defendant’s results. This is common where database and other records are retained. Generally, these issues go to the weight and not admissibility of the evidence. Appropriate analytical protocols with a clear presentation of the basis for confident results in court will generally overcome these issues.
Many legal challenges are overcome by the prepared, clear and honest expert testimony of the forensic scientist. Expert forensic testimony that explains clearly the basis for confidence emerging from an analytically sound program is very persuasive to the courts.
Future Forensic Considerations
Forensic breath alcohol programs today are encountering increased public attention along with added legal challenge and complexity. Protocols that minimize operator involvement and incorporate error detection capabilities should enhance confidence when facing legal scrutiny. As legal per se concentrations decrease, levels of detection and quantitation must be evaluated for specific instruments and testing protocols. All program elements must be continually evaluated to insure forensic integrity.
The forensic application of breath alcohol analyses must continually monitor and thoughtfully consider the relevance of advancing technology. Many aspects of ‘intelligent measurement’ will find important and useful applications in breath alcohol instrumentation. The flexibility of computerized instruments allows for: (1) monitoring simulator standard values and duplicate test agreement; (2) adjusting for known bias; (3) computing confidence intervals; (4) requesting additional samples depending on specified criteria, etc. Many features of ‘intelligent measurement’ can enhance the forensic integrity of breath alcohol results.
Improving analytical specificity for ethyl alcohol will continue to be an important motivation in the future. This issue will likely be addressed from both instrumental and legal perspectives. Multiple infrared filters and dual technology illustrate instrumental responses to the issue while both statutory revision and case law will contribute to the legal perspective. Moreover, further research will be necessary to determine the biological and kinetic details following exposure to volatile organic compounds and the likelihood of even having measurable levels in the breath of drunk drivers.
The analytical process should become as direct as possible while attempting to measure exactly what is defined in the law. Statutes that prohibit specific breath alcohol concentrations simplify and improve the analytical process and interpretation. On the other hand, laws attempting to improve the estimation of blood alcohol equivalent concentrations by measuring and correcting for breath temperatures appear only to complicate and add considerable uncertainty to the process.
The need may arise to formulate a legal definition of ‘breath’ for forensic purposes. A person may be above or below the per se limit depending on length and technique of exhalation. The inherent variability of alcohol concentration in a single exhalation has prompted legal debate in this regard. Instrumental sampling features may have to be combined with a legal definition to clarify the issue.
The ultimate purpose of forensic breath alcohol measurement is to present reliable information that will facilitate an informed decision consistent with the relevant law. Future efforts must be directed toward improving this communication process. The use of visual aids, selected analogies, simplifying terms, and honest clear presentation will help to avoid confusion with technical material. The informative transformation of measurement results into clear and relevant terms must be a continual motivation for the forensic scientist who communicates and interprets breath alcohol results.
