Introduction
Forensic science is applied science. That is to say that the scientific methodologies used in forensic science were developed by biologists, chemists and geneticists and then taken over by forensic scientists to help them solve problems. Research in the 1950s, 1960s and 1970s identified polymorphic enzymes such as acid phosphatase (ACP, referred to as erythrocyte acid phosphatase (EAP) by forensic scientists), adenosine deaminase (ADA), adenylate kinase (AK), carbonic anhydrase (CA2, only useful with samples of African American origin), esterase D (ESD), glyoxalase (GLO), peptidase A (PEPA, primarily of use with samples of African American origin), and phospho-glucomutase (PGM1) and proteins such as group specific component (GC, now known as Vitamin D binding globulin (VDBG)), hemoglobin (HB, primarily used with samples of African American origin), haptoglobin (HP), the immunoglobulin allotypes (GM and KM), and transferrin (TF). These markers were used in the late 1970s and early 1980s by forensic science in the United States and abroad to individualize bloodstains. Although some of these markers did not last long in bloodstains they made it possible to individualize bloodstains with greater than a 99% certainty. Semen stains, saliva and urine had relatively few markers that could be detected, so it was often difficult to provide information in cases with these types of evidence. The ABO blood groups (Secretors), Lewis blood group, immunoglobulin allotypes, and the enzyme PGM1 were the markers routinely used in these cases, with PEPA and GLO occasionally useful.
In 1980 with the identification of the first hyper-variable DNA polymorphism (D14S1), detected by restriction length polymorphism technology (RFLP), the door was opened to the possibility that DNA technology could be applied to forensic evidence. In the next several years, the search for new markers led to the identification of many forensically useful markers, detected by RFLP, some of which are still used routinely in forensic DNA testing. In the mean time hypervariable minisatellite regions were found which identified many genetic regions at one time (multi-locus probes). The term ‘DNA fingerprinting’ was used to describe these bar code like patterns. Although these regions proved to be highly informative for parentage testing, they did not have the sensitivity needed for forensic testing. Though multi-locus probes are used outside of the United States for paternity testing and some forensic applications, they were never widely accepted in the United States.
At the same time as the revolution in RFLP was beginning in the mid 1980s, the development of modern polymerase chain reaction (PCR) tests occurred. The role of the polymerase enzymes in copying DNA had been known since the 1970s. The use of high temperature Taq polymerase allowed for the automation of thermal cycling and the introduction of modern PCR. Tests were developed to identify human leukocyte antigens (HLA) for the transplantation community.
The first marker to be developed was to the HLA region called DQa (now called DQA1). This became the first PCR-based test to be used forensically.
In the mid 1980s the only DNA RFLP testing on forensic evidence available in the United States was through two commercial laboratories. Initially DNA RFLP testing was only done on cases that could not be resolved by non-DNA enzymes and protein testing. The initial success rate was low, approximately 10%, largely due to the nature of the evidence tested. As the success rate for testing sexual assault evidence improved so did the demand for testing. The FBI began to develop procedures of RFLP typing and establishing a standardized system for publicly funded crime laboratories in the United States. Similar work was going on in Canada, the UK and Europe. Initially, different restriction enzymes were used. The Lifecodes Corporation used the enzyme Pst I; Cellmark used the enzyme Hinf I; and the FBI chose the enzyme Hae III. The United States and Canada standardized using a forensic RFLP-system based on the enzyme Hae III whereas England and Europe standardized by using the enzyme Hinf I. In the late 1990s all forensic DNA testing using RFLP is done using either the restriction enzyme Hae III or Hinf I.
What is DNA?
DNA stands for deoxyribonucleic acid. It is the biological blueprint of life. DNA is made up of a double-stranded structure consisting of a sugar (deoxyribose) and phosphate backbone, cross-linked with two types of nucleic acids referred to as purines (adenine and guanine) and pyrimidines (thymine and cytosine) (Fig. 1). The cross-linking nucleic acids always pair a purine with a pyrimidine, such that adenine always pairs with thymine and guanine always pairs with cytosine.
DNA can be found in several areas of a cell. The majority of DNA is located in the nucleus (Fig. 2) organized in the form of chromosomes (22 pairs of autosomes and a set of sex chromosomes (X and Y)). Each nucleated cell normally has 46chromosomes that represent the contribution from both parents. In the formation of gametes (eggs and sperm) one chromosome of each pair is randomly separated and placed in the gamete. The separation of chromosomes is referred to as segregation. The transmission of half of our chromosomes to our children in the form of gametes is the basis of Mendelian inheritance. This DNA is referred to as nuclear or genomic DNA. With the exception of identical twins, no two people share the same genomic DNA sequence.
Another source of DNA is found in the mitochondria in the cytoplasm of cells (Fig. 2). Unlike nuclear
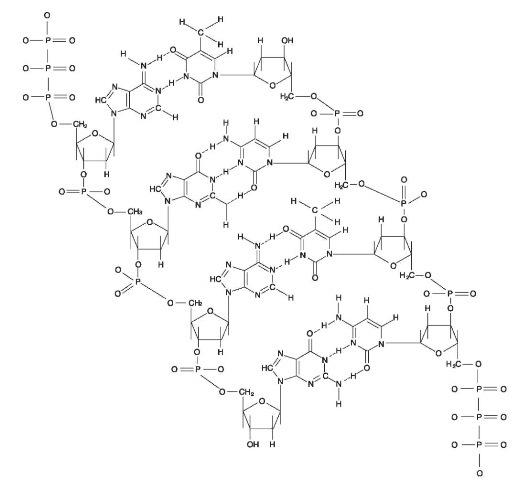
Figure 1 Molecular structure of DNA. From top to botttom: Adenine-Thymine, Guanine-Cytosine, Adenine-Thymine and Guanine-Cytosine.
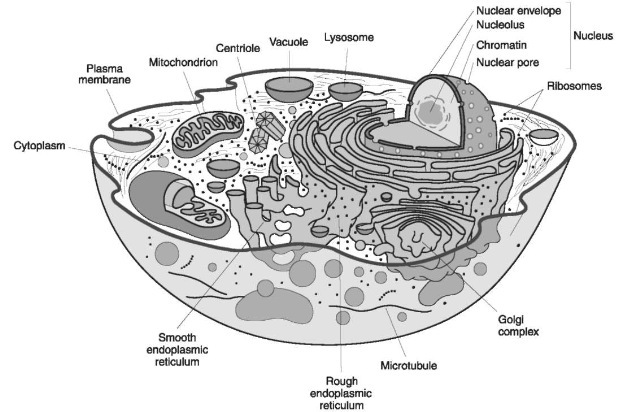
Figure 2 A generalized eukaryotic cell showing the organization and distribution of organelles as they would appear in transmission electron microscope. The type, number and distribution of organelles is related to cell function.
DNA, which only has two copies of each genetic region, mitochondrial DNA is involved in energy production within the cell and can have between 100 and 10 000 copies per cell. Structurally, instead of a linear arrangement of DNA within chromosomes, mitochondrial DNA has a circular structure. Mitochondrial DNA is inherited from the mother because it is found in the cytoplasm which comes from the egg (ova).
Where is DNA Found?
Nuclear or genomic DNA is found in all nucleated cells as well as in the reproductive cells (eggs and sperm). The amount of DNA we can expect to find in different cells and types of evidence is found in Table 1. DNA has been successfully obtained from blood and bloodstains, vaginal and anal swabs, oral swabs, well-worn clothing, bone, teeth, most organs and to some extent urine. It is less likely that DNA will be obtained from some types of evidence than others. Blood or semen stains on soil and leather are historically not good sources of evidenciary DNA. Saliva per se has few nucleated cells, but, beer and wine bottles, drinking glasses, beer cans, soda cans, cigarettes, stamps and envelope flaps have all been found to provide varying amounts of DNA.
How much DNA is Needed for Forensic Testing?
The amount of DNA needed to perform testing depends on the technology used. RFLP technology usually needs at least 50 ng of intact high-molecular-weight DNA. In contrast PCR-based testing can use as little as 500 pg. Most PCR-based tests are set up to use between 1 and 10 ng of genomic DNA.
Destruction of DNA
Biological materials are affected by their environment. Enzymes lose activity over time and type of storage conditions. DNA has been found to be relatively robust when it is in the form of dry stains. Initial environmental studies indicated some of the limitations of DNA based on the material it is deposited upon and the environmental conditions. Environmental insult to DNA does not change the results of testing, you will either obtain results, or if the DNA has been too badly affected by the environment (i.e. the DNA is degraded) you do not get RFLP results. One report on the success rate of obtaining RFLP results noted that depending on the substrate or condition of the stain, results were obtained between 0 (carpet stains or putrefied samples) and 61.5% (scrapped dried stains) of the time with an average of 52% for the 100 items of evidence tested. Thus, the material that the DNA is deposited on and the degree of further insult can markedly affect the ability to obtain RFLP DNA results.
Table 1 DNA content of various tissues
| Isperm | 3pg |
| 1cell | 6pg |
| 1shed hair | 1 nga |
| 1plucked hair | 300 ngfe |
| 1drop of blood | 1500 ng |
All the published studies on environmental insult were done on prepared dried stains. Since biological fluids are liquid the effects of ultraviolet radiation on liquid DNA have been evaluated. The results of exposing 100 ul samples of a standard DNA solution to fluorescent light in the laboratory, a UV germicidal light (254 nm), mid day sunlight in January, and early sunset light in January in 15 min increments, up to 1 h are presented in Fig. 3. There is a linear decrease in high-molecular-weight DNA with the UV germicidal light, such that after an hour about 96% of the high-molecular-weight DNA has been lost. Even in the weak mid day light in January, over 60% of the high-molecular-weight DNA was lost. In contrast, the fluorescent lighting in the laboratory and the after sun set light had no effect on the amount of high-molecular-weight DNA. This was not a rigorous experiment, but the effects are dramatic enough to demonstrate the effect of ultraviolet light exposure to DNA before stains dry.
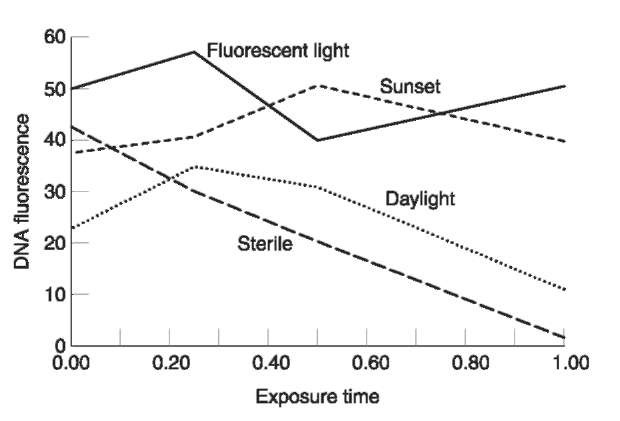
Figure 3 Plot of DNA concentration (flourescence) over time, after exposure to different light sources.
Extraction of DNA
As stated previously DNA exists inside cells. Because most evidence is in the form of dry stains, the DNA must be removed from the stain before it can be tested. The process of removing DNA from the cells on the evidence and dissolving it is referred to as extraction. There are several procedures available for removing DNA from evidence so that it can be used. They are referred to as either ‘organic’ extraction or ‘nonorganic’ extraction based on the nature of the chemicals used. Further, there are two special types of extraction. The first, called differential extraction, was developed for sexual assault evidence to separate the cells that come from the victim (epithelial cells from the vagina, rectum or mouth) from those of the perpetrator (male sperm cells). The second method is a specialized ‘nonorganic’ extraction using Chelex beads. Chelex beads can only be used when PCR-based DNA testing is going to be used. The basic DNA extraction procedures, whether organic or non-organic, can be adapted for special circumstances such as hair or tissue.
Chloroform-phenol extraction
This is the oldest procedure available for extracting DNA from blood and it has been extended to include hair, tissue and semen stains. The basic procedure consists of opening up cells with a buffer and an enzyme, usually protease K, and then denaturing and separating the proteins from the DNA. The latter part is done using a mixture of chloroform (chloro-form:isoamyl alcohol 24:1) and phenol (buffered). The phenol-chloroform mixture denatures proteins liberated by the first stage. The major disadvantage of this procedure is the fact that phenol-chloroform is a hazardous waste and could theoretically pose a risk to pregnant employees. A modern protocol for phenol-chloroform extraction of various types of evidence can be found in the literature.
Nonorganic extraction
In nonorganic extraction the hazardous phenol-chloroform protein denaturation step is replaced by a salting out of proteins. This allows for the same chemistry to be used for the initial phase of DNA extraction, and replacement of the hazardous elements of the procedure with a nonhazardous alternative. The salting out procedure has several advantages over the phenol-chloroform extraction. The first is that instead of having two liquid phases (organic and nonorganic) that can occasionally trap the DNA in the wrong (organic) phase, by precipitating the proteins (e.g. the proteins become insoluble and become a solid), there are liquid and solid phases with the DNA only in the liquid phase (nonorganic). The second advantage is that the hazardous phenol-chloroform is replaced with a harmless salt solution. Comparison of the organic and nonorganic procedures for blood and semen indicate that the nonorganic extraction is on the average as good as or better than organic extraction, whether quantitated by yield gel or slot blot (Table 2).
Either method of DNA extraction described above can be used for both RFLP or PCR-based DNA testing. Organic DNA extraction is widely used in laboratories doing criminal case work whereas non-organic DNA extraction is widely used in laboratories performing paternity testing, research and diagnostics. On a worldwide basis nonorganic DNA extraction is the more prevalent. With the shift to PCR-based testing this choice in extraction is becoming increasingly common.
Table 2 Comparison of organic and nonorganic extraction of DNA from blood and semen stains
| Quantitation method | Blood | Semen | |
| Organic Nonorganic | Organic | Nonorganic | |
| Yield gel | |||
| Mean | 185 ng 258 ng | 175 ng | 207 ng |
| N | 218 | 22 | 8 |
| P | 0.054 | 0.122 | |
| Slot blot | |||
| Mean | 515 ng 908 ng | 627 ng | 1175 ng |
| N | 22 8 | 27 | 8 |
| P | 0.022 | 0.008 |
Chelex extraction
In 1991 a method of DNA extraction was described that was specifically aimed at the extraction of small amounts of dilute DNA for PCR-based testing using Chelex beads. The method is simple, relatively fast and biohazard free. It is widely used by forensic laboratories doing PCR-based typing which has increased the number of laboratories using nonor-ganic, biohazard free DNA extraction. The only limitations of Chelex extraction is that it produces a dilute solution of DNA that may need to be concentrated before it can be used with some of the newer high resolution PCR-based typing systems.
Quantitation of DNA
Yield gel quantitation
Whether RFLP- or PCR-based testing is performed it is necessary to know how much DNA is present. One of the earliest methods of quantitating small amounts of DNA is the use of a yield gel. A small gel is made using a salt solution to carry electrical current and a supporting medium made of agarose (a complex carbohydrate made from seaweed). Much like gelatin, the agarose is dissolved in water that is heated to near boiling, the liquid is cooled slightly and poured into a forming tray. A plastic comb or former with rectangular teeth is placed in the liquid agarose. Once the agarose gels, the comb is removed leaving behind rectangular wells in the agarose gel. The DNA to be tested is mixed with loading buffer, and placed in the wells. Loading buffer is a mixture of a large amount of sugar and dye. The high concentration of sugars makes the mixture heavier than the salt solution so that the DNA sinks to the bottom of the well. The dye allows the migration of the DNA to be monitored. As the agarose was melted in water containing salt, when an electrical current is applied to the gel, electricity flows through the gel because of the salt and moves (migrates) from the negative electrode (cathode), toward the positive electrode (anode). Since all DNA has a negative charge, and was placed in the wells at the cathodal end of the gel, the negatively charged DNA will migrate out of the wells toward the positive end of the gel. If the DNA is broken into pieces that are different sizes, the smaller pieces will move through the gel faster than the larger pieces and will be separated based on size. This process of separating DNA using an electric current is called electrophoresis, which simply means separation (phoresis) by means of electricity (electro).
Since DNA is colorless it is not possible to see the DNA after it has been separated without the use of special dyes that bind to it. One of the earliest dyes used was ethidium bromide which fluoresces pink when bound to double-stranded DNA and exposed to ultraviolet light. Fig. 4 is an ethidium bromide-stained yield gel. To quantitate the amount of DNA in the DNA extracts, a set of DNA quantitation standards are placed on the gel. By visual comparison of the unknown DNA with the known DNA the amount of DNA can be approximated. This test provides information about the relative amount of DNA and whether it is degraded (i.e. the DNA is broken down so that different size pieces of DNA are present). It does not indicate if the DNA is human, however, since all DNA will fluoresce. Thus the DNA present may be bacterial as well as human DNA. For RFLP testing the total amount of DNA in the sample is the important determinant of how the samples migrate in the gel. Therefore, yield gel electrophoretic quantita-tion of DNA is an appropriate method. Yield gel quantitation of DNA for RFLP testing was considered to be such an integral part of quality assurance that it was included in the National Institute of Standards, Standard Reference Material 2390, ‘DNA Profiling Standard.’
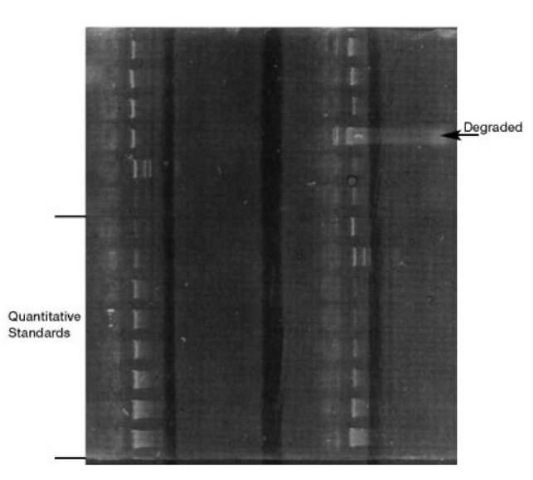
Figure 4 Ethidium bromide stained yield gel. Bottom left samples are quantitative standards. Other samples represent various samples of DNA. Upper right sample is degraded DNA.
Slot blot quantitation
In contrast to RFLP, for PCR-based testing, the amount of human DNA and not the total amount of DNA is an important determinant in how likely it will be to obtain results. A slot blot does not rely on elec-trophoresis to separate the DNA but rather on the ability of denatured (separated DNA strands) DNA to bind to homologous complementary sequences. The ability to quantitate human DNA requires sequences of DNA that are common in the human genome so that a single DNA sequence can recognize them and bind them. The repeated DNA sequence called D17Z1 is the basis for all human DNA slot blot quantitation systems. There are several of these procedures commercially available. In one of the most widely used tests, the quantitation requires that denatured DNA is applied to a membrane using a slotted plastic apparatus. The denatured DNA binds to the membrane. The membrane is exposed to a solution of denatured DNA fragments that recognizes a repeating sequence of human or primate DNA. Pieces of DNA that recognize a specific region of DNA are referred to as a ‘probe’. The probe will bind to complementary DNA fragments stuck on the membrane. The probe has an indicator attached to it so that the binding of the DNA to the probe can be detected. The unbound probe is washed off and the probe is detected using either chemicals that change color (colorimetric detection) or chemicals that give off light (chemilu-minescent detection). To be able to quantitate the amount of human DNA present, standards with different amounts of human DNA are also placed on the membrane. A series of known DNA quantitation standards are included in the blot so that it is possible to determine the approximate amount of DNA bound to the membrane by visual comparison to the known standards. More precise quantitation can be obtained by scanning the membrane with a scanning densit-ometer and determining the amount of color associated with each band. Most forensic laboratories use visual comparison.
