Caution!
Some explosives are extremely sensitive to sparks, heat, shock or friction; necessary precautions should therefore be taken by the analyst while handling explosives.
Introduction
General
An explosive is defined as a substance or mixture of substances which may be made to undergo a rapid chemical change without an outside supply of oxygen, with the liberation of large quantities of energy, generally accompanied by the evolution of hot gases.
An explosive mixture must contain an oxidizing agent and a reducing agent.
Explosives are used legally in industry and in the military but also illegally in terrorist and criminal activity. Some explosive compounds have other uses such as in medicine (some nitrate esters) or in the paint industry (cellulose nitrate).
Military and industrial explosives are routinely analyzed after their production for quality control. In this area, apart from qualitative analysis, the analysis usually includes quantitation of explosives, byproducts and impurities in order to meet accepted specifications. Other areas which deal with the analysis of explosives are forensic science, environmental analysis and in certain cases, toxicology.
Forensic analysis of explosives deals with the identification of unreacted explosives as well as with postexplosion identification. The identification of an unexploded explosive is carried out to prove its possession or its intended use.
In postexplosion analysis the situation is different; when an explosion has already occurred, it is reasonable that an explosive was involved. It may seem unnecessary to perform postexplosion analysis, but such analyses have the highest priority in most forensic laboratories. The reason is that information about the explosives involved can be of great assistance to the investigation. Sometimes it is not unequivocally clear whether the explosion was initiated by a high explosive or by the ignition of a fuel-air mixture (‘vapor explosion’). When an explosive is identified in residues it may strongly suggest that it caused the explosion. On the other hand if no explosive is identified it may suggest that no explosive was involved (e.g. ‘vapor explosion’) but it may also be that the analysis was unsuccessful. Sometimes the results of the analysis can direct the investigator as to whether the explosion was carried out by terrorists or by criminals unrelated to terrorist activity. Certain types of explosives have been typical to terrorist groups (e.g. ‘Semtex’, especially before the fall of the ‘iron curtain’). In rare cases the type of explosive may even hint to a certain organization. Another reason to pursue postexplosion analysis is the need for law-enforcement agencies to know what materials are used by criminals or terrorists. This information may help in connecting between different cases and also to realize that some materials are not as ‘innocent’ as they seem, but are starting materials for the preparation of these explosives (e.g. acetone and hydrogen peroxide for the preparation of triacetonetriperoxide (TATP)).
Another very important type or work is the trace analysis of explosives on suspects’ hands, and on items and premises which may be related to suspects. Although not a postexplosion situation, the procedures used in such analyses are similar to those used in postexplosion analysis. The analysis in these cases is difficult because it usually deals with trace amounts of unreacted explosive mixed with large amounts of contaminants. The ultimate goal of a forensic analyst is to provide an expert opinion for a court of law. Wrong results may lead to a gross injustice, where innocent people can be found guilty. This dictates the need to adhere to extremely strict criteria for the safe identification of explosives.
Classification of explosives
Many compounds, which may be classified as explosives, are listed in the literature. In practice, the forensic analyst routinely encounters only few compounds. Explosives may be classified according to their chemical structure, use, their place in the detonation chain and their explosive properties. These widely used classifications are given, along with a few examples:
Chemical structure
• Organic nitro explosives:
Nitroaromatic: 2,4-dinitrotoluene (2,4-DNT), 2,4,6-trinitrotoluene (TNT), picric acid; Nitrate esters: ethyleneglycol dinitrate (EGDN), glycerol trinitrate (NG), pentaerythrithol tetra-
nitrate (PETN), cellulose nitrate (NC); Nitramines: 1,3,5-trinitro-1,3,5-triazacyclohex-ane (RDX), 1,3,5,7-tetranitro-1,3,5,7-tetraza-cyclooctane (HMX);
• Organic peroxides: 3,3,6,6,9,9-hexamethyl-1,2,4,5,7,8-hexaoxacyclononane (TATP), 3,4,8,9,12,13-hexaoxa-1,6-diazabicyclo [4,4,4] tetradecane (HMTD);
• Inorganic salts: ammonium nitrate (AN);
• Mixtures of oxidizing and reducing agents: black powder (potassium nitrate, sulfur and charcoal), potassium chlorate and sugar.
Structural formulae of organic explosives representing each chemical group are shown in Fig. 1.
Use
• Military explosives: TNT, PETN, RDX
• Industrial or commercial explosives: dynamites, AN, emulsion explosives.
• Illegally manufactured improvised explosives: TATP, potassium chlorate and sugar mixture.
Place in detonation chain
• Primary explosives: mercury fulminate, lead styph-nate, lead azide, dinol.
• Boosters: PETN.
• Main charge: TNT, RDX.
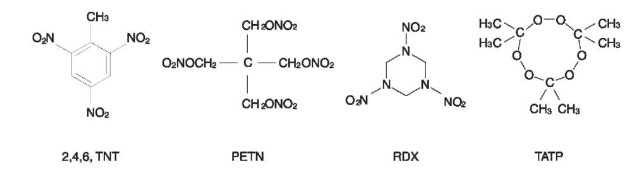
Figure 1 Structural formulae of some organic explosives.
Explosive properties
• High explosives: PETN, RDX
• Low explosives (propellants): smokeless powder, black powder.
Explosive formulations usually contain other compounds such as sensitizers, desensitizers, stabilizers (e.g. diphenylamine (DPA), ethyl centralite (EC)), plasticizers (e.g. phthalate and sebacate esters) and other additives.
Methods and Procedures Analytical methods
Various analytical methods have been used for the analysis of explosives: chemical tests (based on color reaction); chromatographic methods such as thin layer chromatography (TLC), column chromatography, gas chromatography (GC), high pressure liquid chromatography (HPLC), capillary electrophoresis (CE) and ion chromatography (IC); and spectral methods such as infrared (IR), nuclear magnetic resonance (NMR), mass spectrometry (MS), scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM/EDX) and X-ray diffraction (XRD). Hyphenated methods, usually on-line combinations of chromatographic and spectroscopic methods (e.g.
GC/MS, HPLC/MS) are powerful analytical techniques which have become widely used in forensic laboratories.
Analytical procedures
The analytical procedures used in forensic laboratories for the analysis of explosives are based on the methods described above. Naturally, a procedure for postexplosion analysis is different from the analysis of intact explosives.
In the latter case the analysis is based on a normal methodology for unknown samples. The procedure may include spot tests, chromatographic methods and spectrometric methods. It is essential for the forensic laboratory to have a library of spectral data of all common explosives and related compounds and preferably also authentic samples of the explosives. The analysis usually includes identification of the sample but sometimes quantitation is also required (e.g. when a common origin of samples is suspected).
Procedures for postexplosion analysis are much more complicated, including recovery techniques as well as methods of identification. Recovery methods may include microscopic examination, headspace sampling, adsorption and extraction procedures. The identification, as in many areas in forensic analysis, may start with a screening method followed by a suitable confirmation method. Postexplosion analysis, including a flow chart, is discussed separately later.
The methods in a procedure may also be derived from the properties of the explosives. Different explosives have different chemical and physical properties. Some explosives are highly volatile (e.g. EGDN), some undergo easy sublimation (e.g. TATP) and some are nonvolatile (e.g. HMX). Some explosives are thermally labile and decompose when heated. Knowing the properties of the different explosives is relevant to the choice of the most suitable method for the recovery and the analysis of specific explosives. For example, analytical methods which involve evaporation of compounds (e.g. GC or GC/MS) may be unsuitable for nonvolatile explosives.
Finally and not less important, the procedures used in each laboratory are influenced by the financial ability to purchase sophisticated instrumentation and by the professional skill of the staff.
Methods, advantages and limitations
Chemical tests Chemical tests, also referred to as spot tests or color tests, take advantage of a color produced by a reaction between a reagent and an analyte. Some well-known color reactions (sometimes in a modified version) are used in the analysis of explosives. These color reactions are widely used by many forensic laboratories as presumptive tests, for screening and for field tests.
Some widely used spot tests are described according to the classes of explosives. Di- and trinitroaro-matic compounds develop colors with some basic solutions: TNT develops a purple-brown color whereas 2,4-DNT and 2,6-DNT develop a yellowish color when reacted with KOHin ethanol (e.g. 3%). The colors developed in the reaction between poly-nitroaromatic compounds and bases are sometimes attributed to the formation of the so called ‘Meisen-heimer complexes’.
The Griess reaction is a well-established, highly specific color reaction for the identification of nitrite ions. In this reaction, nitrite ion reacts with an aromatic amine, such as sulfanilamide, in an acidic medium to form a diazonium ion. This ion is then coupled with a suitable active aromatic compound, such as N-l-naphthylethylenediamine, to produce an azo compound, which has a characteristic purple color. Nitrate esters and nitramines produce NO2~ ions by the action of an alkali whereas nitrate ions produce the nitrite ions by reduction (e.g. by zinc powder).
The Griess reaction is illustrated in Fig. 2.
The reactions mentioned above are the basis of some commercially available kits, often used in border-crossings to screen items or persons suspected of having been in contact with explosives or materials suspected as explosives. An example is the ETK, an Israeli-produced kit, which is capable of detecting some polynitroaromatic compounds, ester nitrates, nitramines and inorganic nitrate salts. The ETK has been successfully used in real cases.
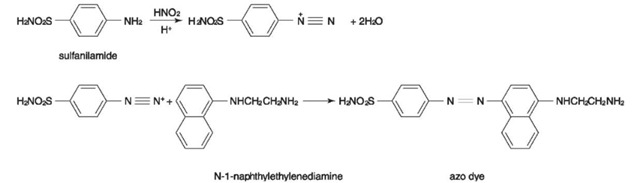
Figure 2 The Griess reaction.
Another type of spot test is based on the oxidation of a reagent by an explosive or an oxidizing constituent of an explosive mixture. Diphenylamine develops a blue color when reacted with TATP; aniline sulfate develops a blue color when reacted with chlorates.
In general, the sensitivity of many spot tests used in the analysis of explosives is in the microgram range. Spot tests are fast, inexpensive, simple, do not need instrumentation and may be performed by technicians in the field. Spot tests cannot be the basis for reliable identification. At best they may strongly indicate that the analyte belongs to a certain class of compounds (e.g. nitroaromatic).
Separation methods Separation methods are used to separate a mixture into its components. Almost all separation methods used nowadays are chromato-graphic methods. These methods utilize differences in the affinity of the components of a mixture to stationary and mobile phases. Chromatographic behavior of a compound in given stationary and mobile phases is expressed by its migration rate, usually called rate of flow (Rf) or retention time (Rt). The retention data may also depend on other conditions such as temperature (e.g. in GC). Though originally used to separate a mixture, chromatographic methods have often been used to tentatively identify a compound by comparing its retention data to those of a known compound. This is best done by analyzing the analyte and the authentic sample under the same conditions. Chromatographic methods are used in forensic analysis of explosives, both for separation of mixtures and for preliminary identification. Some of the more widely used methods are described below.
TLC In TLC the stationary phase consists of an adsorbent such as silica gel, coated on aluminum or glass plates. Solutions of samples are spotted near one end of the plate which is then placed in a chamber containing the developing solvent (the mobile phase). The developing solvent is allowed to ascend to a certain distance on the plate. Different compounds are carried by the solvent with different rates, resulting in a separation. The plate is then removed from the container and dried. Visualization of the spots is done by ultraviolet (UV) light or by spraying reagents, usually based on the spot tests mentioned above. Both the RF and the color developed by spraying, if similar to those of an authentic sample, may indicate the identity of a compound. TLC can also be used in a preparative mode in which the separated compound is extracted and its identity confirmed by other methods. The sensitivity of TLC is generally in the micro-gram to submicrogram range depending on the type of visualization used. TLC is widely used for the analysis of explosives in forensic laboratories.
Although there are many reports describing different TLC systems for the separation of explosive compounds, no single system has been reported to separate all organic explosives. Therefore a combination of several systems is routinely used, such as: (l) l,2 dichloroethane:acetonitrile (90:10, v/v); (2) tri-chloroethylene:acetone (80:20, v/v); (3) petrol ether (b.p. 60-80):ethyl acetate (90:10, v/v). Figure 3 shows some military explosives separated by these TLC systems.
A TLC plate, sprayed with the reagents mentioned above deteriorates quickly; therefore it is recommended that documentation by camera or scanner is carried out immediately after spraying.
TLC is a simple, inexpensive and fast method allowing analysis of several samples in a single run. However, it is considered a low-resolution method and in addition, separation is susceptible to contaminants (when present in high amounts). It must be emphasized that identification cannot be based on TLC only and must be confirmed by other methods.
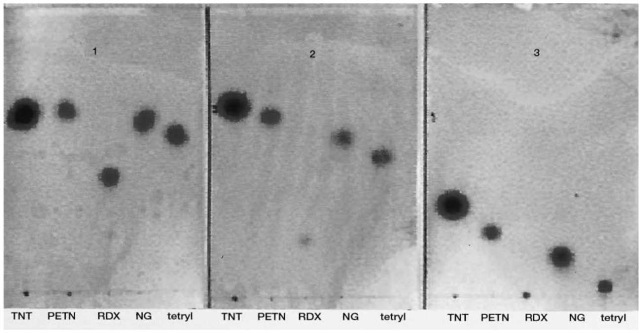
Figure 3 Military explosives (TNT, PETN, RDX, NG and tetryl) separated on three TLC solvent systems: (1) 1,2 dichloroethane:acetonitrile (90:10); (2) trichloroethylene:acetone (80:20); (3) petrol ether (b.p. 60-80):ethyl acetate (90:10).
GC Modern GC utilizes capillary columns in which the stationary phase is chemically bonded to the fused silica wall. A sample, dissolved in a suitable solvent, is injected into a heated injection port. The compounds are evaporated and pushed along the coated column, usually at elevated temperatures, towards a detector by the flow of a mobile phase (carrier gas, e.g. helium). Different compounds, with different affinities to the stationary phase, are separated, usually with a good resolution. GC is also a suitable method to perform quantitative analysis.
A typical column used in the analysis of explosives is the nonpolar diphenyl (5%)-dimethylsiloxane (95%) copolymer (e.g. DB-5, HP-5, SP-5), 25 urn coating, l5 m long. Typical GC conditions are: injector temperature 180°C, column temperature programmed from 50°C to 250°C at a rate of 25°C min-1. GC is a simple, high-speed and high-resolution method that is very suitable for the analysis of nitroaromatic explosives. Some loss in sensitivity is observed when analyzing thermally labile compounds (e.g. some nitrate esters and nitramines). Sometimes decomposition products are produced in the injector or in the column. Nonvolatile explosives (e.g. HMX, inorganic compounds) cannot be analyzed by GC. The organic peroxides TATP and HMTD can be analyzed by GC.
Several common detectors are used in GC.
Flame ionization detector (FID) FID, a very common detector in GC used in forensic laboratories, is less common in the analysis of explosives. This is mainly due to a decreased sensitivity towards some explosives which have a high O/C and N/C ratios in their molecules (e.g. NG).
Electron capture detector (ECD) In ECD the eluent from the GC column passes through a slow-electron beam. An analyte containing electronegative atoms such as nitrogen, ‘captures’ electrons from the constant electron current, producing a signal by decreasing this current. Sensitivity is usually in the picogram range. ECD is quite selective, as it is highly sensitive towards nitrogen-containing compounds but insensitive to hydrocarbons.
Chemiluminescence detector (thermal energy analyser (TEA)) In this detector the eluent from the GC column passes through a furnace which pyrolizes the compounds at elevated temperatures (e.g. 500°-900°C). Nitro and nitroso compounds produce nitrogen oxide which is then allowed to react with ozone to produce nitrogen dioxide in an excited energy level. Decaying of the excited NO2 to its ground state is accompanied by emission of light at the IR region which is monitored by a suitable detector. Sensitivity is in the picogram range. Commercial Instruments (e.g. ‘Thermal Energy Analyser (TEA)’) are available and are very sensitive and highly specific for the analysis of nitro-containing compounds (though there have been some reports of signals from compounds without nitro or nitroso groups). GCTEA is suitable for the analysis of postexplosion residues mainly because most contaminants having no nitro groups, are not observed. GC/TEA is therefore widely used in the forensic analysis of explosives. The TEA detector is expensive, and its use is limited to the analysis of nitro-containing explosives.
MS This is discussed in more detail below.
HPLC In HPLC the stationary phase is often a ‘reversed phase’ type, such as octadecylsiloxane (ODS). The mobile phase is a solvent or a mixture of solvents which is pumped into the column at relatively high pressure, usually at room temperature. HPLC may be also used in a preparative mode by collecting the desired fraction and confirming its identity by other methods. Some widely used detectors in explosive analysis are discussed below.
Ultraviolet (UV) and diode array (DA) The eluent from the column passes through a cell irradiated by UV light. In the UV detector the source is set at a fixed wavelength or scanned over the UV range. In the DA, multiwave radiation is applied. The UV spectrum obtained by the DA is easily subjected to a computerized library search. These detectors have a low selectivity but their sensitivity is rather high (in the nanogram range) and they are widely used in many forensic laboratories.
Electrochemical detectors In these detectors the detection is based on the electrochemical properties of functional groups in the analyte. A signal is generated when an analyte exchanges electrons with the electrode, if sufficient potential is applied to it. Detection of explosives have been done using pendant mercury drop electrode (PMDE).
Chemiluminescence detector See above.
MS See below.
Ion chromatography (IC) This method utilizes ion-exchange resin as the stationary phase and a solution of salts as the mobile phase. The functional group attached to the stationary phase in anion analysis is usually a quaternary ammonium ion and in cation analysis the exchange function is usually a sulfonate ion. Some instruments utilize a suppressor reaction which takes place in a second column (‘suppressor column’) situated after the ion-exchange column. This enhances the sensitivity by lowering the background noise of the detector. Sensitivity is in the nanograms range.
IC is widely used in the analysis of explosives and related materials. It is used to separate inorganic ions present in low explosives (e.g. black powder),commercial explosives (e.g. dynamites, ammonium nitrate-based explosives) and home-made explosives (e.g. chlorates).
The common detection modes employed in IC are as follows.
Conductivity detector In this detector a signal is produced when an eluted ion reaches the detector and changes the conductivity of the solution.
UV/VIS Since most inorganic ions do not absorb UV or visible light this detector is used in IC in an indirect mode by adding UV-absorbing eluent to the mobile phase.
Capillary electrophoresis (CE) Chromatography is performed on a capillary column, immersed at its two ends in a buffer solution. SiO- groups are formed near the capillary wall in the aqueous medium, leading to accumulation of solvated cations. Application of an electric field (e.g. 10-25 kV) on the capillary results in the migration of the solvated cations towards the cathode, generating electro-osmotic flow (EOF). Analytes introduced to the capillary column move in different directions and with different mobilities; negatively charged species move towards the anode, positively charged species move towards the cathode and neutral compounds move with the EOF. Since the EOF is usually faster than the migration velocity of the anions, all species are swept towards the detector which is usually situated near the cathode. Compounds with different mobilities are usually separated with a high efficiency because the profile of the advancing mobile phase is flat, in contrast to its parabolic profile in other separation techniques (e.g. GC and HPLC). Detection is usually by UV where the capillary itself serves as the cell.
In CE it is possible to analyze different classes of compounds: organic and inorganic, neutral and ionic. All these compounds can be analyzed by changing the buffer. Sensitivity is in the picogram range.
The most suitable method for the analysis of ions is capillary zone electrophoresis (CZE) which is applied in some laboratories as a confirmation method for results obtained in IC. Figure 4 shows the analysis of some ions found in residues of a pipe bomb.
Neutral organic explosives and other related compounds may be separated by micellar elecrokinetic chromatography (MEKC) in which a surfactant (e.g. sodium dodecyl sulfate (SDS)) is added to the buffer, forming micelles. Separation is achieved by partition of the analytes between the micelles and the buffer. The stronger the attraction between the analyte and the micelle the longer is its migration time.
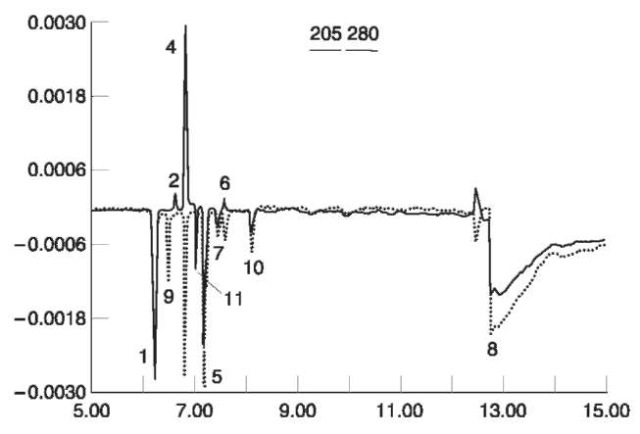
Figure 4 Capillary electropherogram of Pyrodex pipe bomb residue anions. Analysis was performed at 205 and 280 nm. Peak identification:^ Cl-;2,N02-;4,N03-;5,S04-2;6, SCN-;7,Cl04-; 8, HC03-;9,HS-; 10, 0CN-; 11, unknown.
Spectroscopic and spectrometric methods IR Molecules irradiated by infrared light (e.g. 4000500 cm-1) absorb energy at certain wavelengths which correspond to intramolecular vibrations.
Absorbing bands in the range ~4000-1300 cm-1 are usually associated with specific functional groups whereas absorption bands below ~ 1300 cm-1 are usually characteristic of the molecule as a whole. Therefore the region below 1300 cm-1 is sometimes called the ‘fingerprint’ region of the IR spectrum. Modern IR instruments, used by most laboratories are Fourier Transform IR (FTIR). IR may be used to identify a pure compound by comparing its spectrum to the spectrum of an authentic sample. Sensitivity is usually in the microgram range; detection limit may be lowered by using microscope FTIR.
Mixtures may require chemical separation of components before the analysis in order to obtain IR spectra of the pure components. Modern instruments usually contain computerized libraries, in which a mathematical ‘subtraction’ of a spectrum of one component leads to an IR spectrum of a second component. This may reduce the need for chemical separation.
Symmetric and asymmetric stretching vibrations of the NO2 group give rise to two distinct absorption bands, which have a highly diagnostic value. In nitro-aromatic compounds these bands appear at 13901320 cm-1 and 1590-1510 cm-1, respectively. They can be clearly observed in the IR spectrum of 2,4,6-TNT (Fig. 5).
The two NO2 stretching vibrations in nitrate esters appear at 1285-1270 cm-1 and 1660-1640 cm-1, respectively, as can be seen in the IR spectrum of PETN (Fig. 6).
The two NO2 stretching vibrations in nitramines appear at 1310-1270 cm-1 and 1590-1530 cm-1, respectively, as can be seen in the IR spectrum of RDX (Fig. 7).
An IR spectrum of TATP (which lacks nitro groups) is shown in Fig. 8.
Inorganic anions related to explosives also have highly characteristic absorption bands. Nitrate ions absorb at two bands: 1380-1350 cm-1 and 840815 cm-1. Chlorates absorb at 980-910 cm-1, 630-615 cm-1 and 510-480 cm-1.
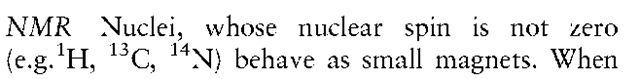
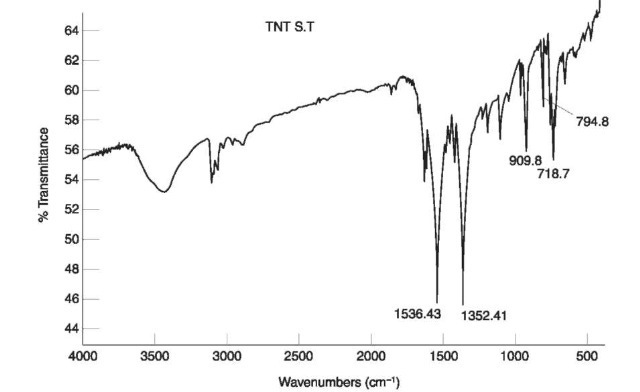
Figure 5 IR spectrum of 2,4,6 TNT.
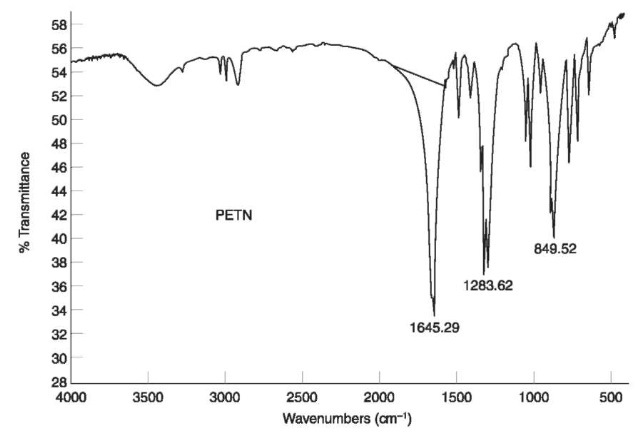
Figure 6 IR spectrum of PETN.
such nuclei are put in an external magnetic field they may align with the magnetic field, having a low-energy orientation, or against it, having a high-energy orientation. Transition between these two energy levels takes place by absorption of suitable radio frequency (RF) radiation called the resonance frequency.
The energy absorbed at such transition depends on the chemical environment of the nucleus; thus, various protons in a molecule resonate at different frequencies. The exact amount of energy absorbed by a specific proton is expressed by its ‘chemical shift’. Different protons in the molecule usually have different chemical shifts. Scanning RF while keeping the magnetic field constant, or scanning the magnetic field while keeping the RF constant will result in an NMR spectrum. The NMR spectrum is highly characteristic and may be used for the identification of a compound by comparing its spectrum to that of an authentic sample. NMR is especially useful for structure elucidation of unknown samples even when no authentic sample is available. This is done by correctly interpreting the different signals in the spectrum, leading, often by combination with other methods, to a complete structure elucidation. Most work in NMR has been done on protons producing databases of chemical shifts and spectra. Sensitivity of NMR is usually in the micrograms to milligrams range. NMR has not been routinely used in the forensic analysis of explosives.
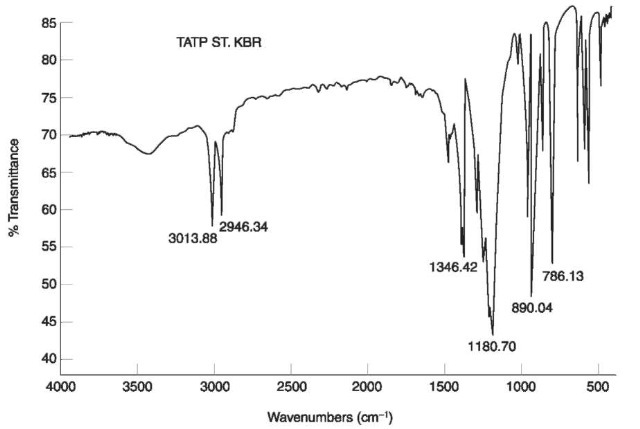
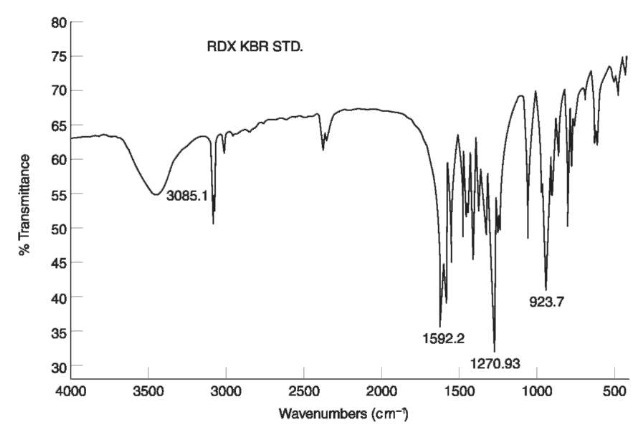
Figure 7 IR spectrum of RDX.
Figure 8 IR spectrum of TATP.
MS In this technique a compound is introduced into an ion source where it is ionized to form molecular and fragment ions according to its structure. The ions pass through an analyzer (e.g. magnet, quadrupole or ion trap) which separates the ions according to their mass to charge ratio (m/z). The ions are detected and recorded, producing a mass spectrum. A mass spectrum often reflects the structure of a molecule. It is usually highly specific and is often referred to as a ‘fingerprint’ of the molecule. Identification of a compound by its mass spectrum is therefore highly reliable. In addition, a mass spectrum can be used for structure elucidation of unknown compounds. Sensitivity usually lies in the picogram to nanogram range depending on the operation mode.
As it is highly reliable as well as highly sensitive, MS is considered to be an excellent method for the identification of organic compounds.
Introduction techniques include inlets for gases and liquids (usually based on needle valves or gold leaks) and direct insertion probe, also known as ‘solid probe’ for solids. Solid probe is usually used for the insertion of nonvolatile compounds, such as HMX, and is usually unsuitable for the analysis of mixtures. However, the most common introduction techniques are the on-line combination with GC (GC/MS) and HPLC (LC/MS).
GC/MS The technical possibility of connecting a highly efficient separation method (GC) with a highly sensitive and reliable identification method (MS) was a breakthrough in analytical chemistry. GC/MS enables the separation of highly complex mixtures with the subsequent rapid identification of each of the separated components. Therefore GC/MS is the method of choice in organic analysis in many forensic laboratories. In modern GC/MS instruments, the GC capillary column end is placed near the ion source without the need of an interface, thus enhancing instrument efficiency. As mentioned above some explosives are easily analyzed by GC (hence by GC/MS) whereas with others some difficulties are encountered.
LC/MS requires an interface between high output of liquids and sometimes nonvolatile buffers, and the high vacuum of the MS. This enables analysis of nonvolatile compounds that cannot be analyzed by GC/MS. The common ionization methods in MS are described below.
Electron ionization (EI): In electron ionization, or electron impact, electrons are ejected from a filament and accelerated usually by a voltage of 70 eV. The sample molecules are bombarded by these electrons, to produce mainly positively charged ions. Printed and computerized databases, containing more than 100 000 spectra, enable a useful library search. Attention should be paid by users to limitations of a computerized search such as the following.
• Computerized libraries may contain a partial mass spectrum of a molecule.
• A library search is performed using a certain algorithm. Using different algorithms may result in
different matches for the same compound, which sometimes may lead to erroneous results.
Therefore, the chemist should always exercise judgment and should not rely only on computer results.
Chemical ionization (CI) In this method the ionization of the sample molecules takes place by reactions with ions derived from reagent gases (e.g. methane, isobutane) present in the ion source at relatively high pressure (~1 torr). This method usually produces [M+H]+ ions, where M is a sample molecule and His the proton transferred from the reagent ions. The [M+H]+ ions have less energy than the molecular ions obtained in EI, resulting in less fragmentation. CI is usually more suitable than EI to obtain molecular weight information and it is best used as a complementary method to EI.
Negative ion mass spectrometry Although less common than mass spectrometry of positive ions, this has some relevance to the analysis of explosives. Formation of negative molecular ions in EI is usually less efficient than formation of the positive ions. In addition, the sensitivity in negative ion MS often depends on the electron affinity of the atoms. CI may also be used in the negative mode, often producing ions which are characteristic of the sample molecules.
Thermospray ionization This term describes both an inlet system and an ionization method for HPLC/ MS. The eluent from the HPLC is rapidly vaporized in a heated metal capillary tube, forming fine droplets. These droplets are ionized by a filament or by electrolyte ions present in the solution. An analyte ion may be generated in the solution and transferred to the gas phase, or generated in the gas phase by ion-molecule reactions. In this method positive and negative ions are formed.
Electrospray ionization (ESI) This is an ionization technique for HPLC/MS or CE/MS which takes place at atmospheric pressure. The eluent from the separation device passes through a stainless-steel capillary to which high positive (or negative) potential (e.g. 35 kV) is applied. This potential leads to the formation of charged droplets. Evaporation of the solvent from the droplets (e.g. by a stream of gas) leaves behind a charged analyte.
Mass spectra Mass spectra of common explosives are discussed below. EI mass spectra of nitroaromatic compounds are highly specific, enabling the differentiation of isomers. A loss of NO2 groups from the molecular ion is an important process, leading to characteristic [M-xNO2]+ ions. Unlike nitrate esters, the abundance of NO2+ ions in the spectra of nitroaromatic compounds is low. The abundant ion (‘base peak’) in the EI spectrum of 2,4,6-TNT (m/z 210) is attributed to an unusual loss of a hydroxyl group from the molecular ion. This loss also occurs in the EI spectrum of other nitroaromatic compounds with a nitro group in an ortho position to a hydrogen-containing moiety such as methyl (‘ortho effect’). The molecular ion is not observed in the EI mass spectrum of 2,4,6-TNT as shown in Fig. 9.
EI spectra of nitrate esters contain highly characteristic ions at m/z 30, 46 and 76, attributed to [NO]+, [NO2]+ and [CH2ONO2]+, respectively. No molecular ions are observed in the EI spectra of these compounds, so the EI spectra of most common nitrate ester explosives are similar, showing ions only at m/z 30, 46 and 76. Therefore, the EI spectrum alone is not sufficient for identification of nitrate esters. CI may serve as a very good confirmation method for these compounds. Their CI spectra contain not only molecular weight information (e.g. [M+H]+ ion), but also characteristic structural information: an abundant ion at m/z [M+H— 63]+ is attributed to the loss of HONO2 from the protonated molecule. EI and CI mass spectra of the two nitrate esters, NG and EGDN, are shown in Fig. 10.
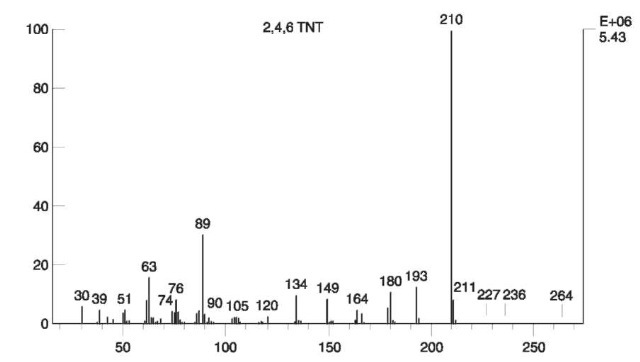
Figure 9 EI mass spectrum of 2,4,6 TNT
EI spectra of nitramines usually lack the molecular ion. The two heterocyclic nitramines RDX and HMX have characteristic EI spectra on which unequivocal identification can be based. The EI spectrum of RDX is shown in Fig. 11 .
Tetryl, a nitramine with a trinitroaromatic nucleus, decomposes in the GC injector in the presence of water, to produce N-methylpicramide. The reaction is shown in Fig. 12. Therefore, the original presence of tetryl should be confirmed by other methods such as IR or TLC which are carried out at room temperature. The EI mass spectrum of N-methylpicramide is shown in Fig. 13.
The EI spectrum of TATP (Fig. 14) contains a low abundant molecular ion at m/z 222.
Other compounds such as plasticizers and stabilizers are usually easily analyzed by GC/MS.
XRD X-ray diffraction, or X-ray powder diffraction (XRPD), utilizes X-ray radiation on crystalline organic and inorganic samples. The rays are diffracted in a pattern determined by the position, arrangement and size of the constituents of the crystal. Scattered photons, which may undergo subsequent interference, lead to a characteristic diffraction pattern. The pattern is characteristic for a specific crystalline powder and may serve as a ‘fingerprint’ of this powder. Identification of a powder may be carried out by comparing its spectrum to a spectrum of an authentic sample. Data bases of diffractograms are commercially available and it is also possible to analyze and identify multiphase crystalline mixtures, qualitatively and quantitatively. Sensitivity is usually in the microgram to milligram range.
XRD is mainly used to analyze crystalline powders of inorganic explosives and explosive mixtures. The advantage of this method over IC or CE is the identification of a compound as a whole; in the latter methods anions and cations are identified separately.
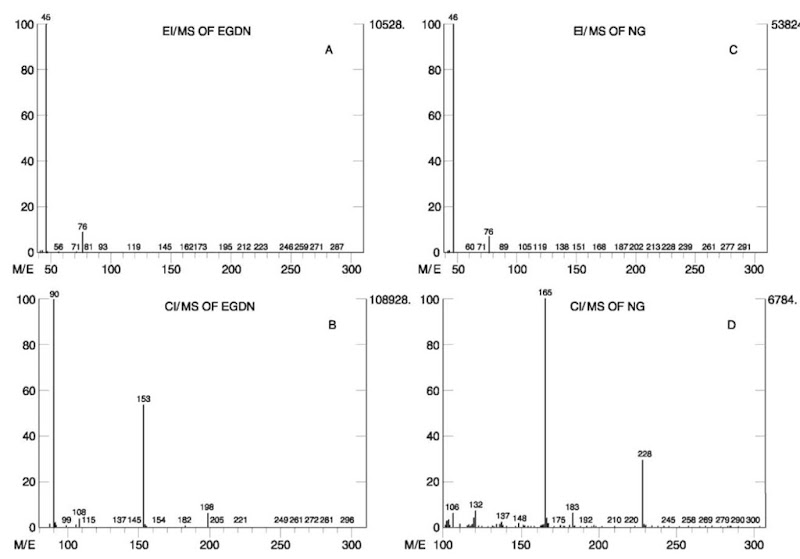
Figure 10 EI mass spectrum (A) and CI mass spectrum (B) of EGDN. EI mass spectrum (C) and CI mass spectrum (D) of NG.
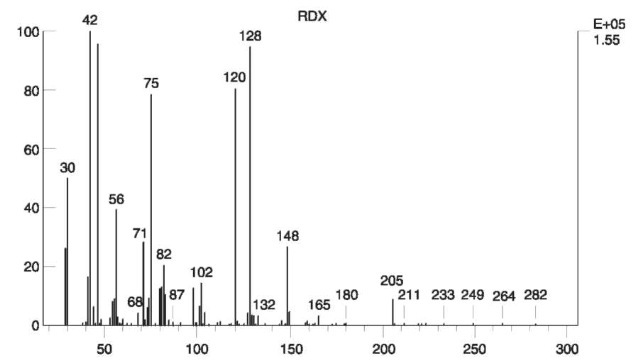
Figure 11 EI mass spectrum of RDX.
XRD instruments are expensive and require expert operators.
SEM/EDX This technique enables examination and morphological characterization of surfaces of organic and inorganic samples. The sample is bombarded by a high-voltage (e.g. 25 kV) electron beam. An interaction between the sample and the electron beam causes emission of radiation in the X-ray range typical of an element. EDX permits high-speed qualitative and quantitative elemental analysis according to the intensity of the energy emitted by the sample. Elements with the atomic number 11 (sodium) and higher may be analyzed by this technique. Special light-element detectors enable the identification of elements with the atomic number 5 (boron) and higher. This has relevance to the identification of explosive compounds which often contain nitrogen atoms. SEM/ EDX is suitable for the identification of metals present in primary explosives such as lead azide or mercury fulminate.
SEM/EDX instruments are expensive and require expert operators.
Postexplosion and Trace Analysis of Explosives
Postexplosion analysis is normally based on the identification of the unreacted explosive which ‘survived’ the explosion. Usually only trace amounts of the unexploded explosives are present. The other alternative, i.e. analyzing decomposition products which are typical of the original explosive, is only carried out in rare cases. An example is the thiocyanate ion (CNS —) whose formation is characteristic of the burning of black powder.
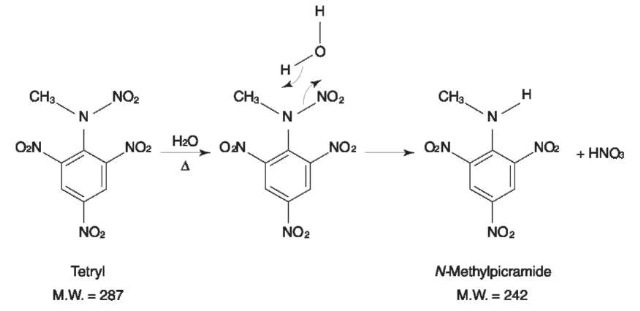
Figure 12 Hydrolysis of tetryl to A/-methylpicramide. Reprinted from Tamiri Tand Zitrin S (1986) Capillary column gas chromatography/mass spectrometry of explosives. Journal of Energetic Materials 4:
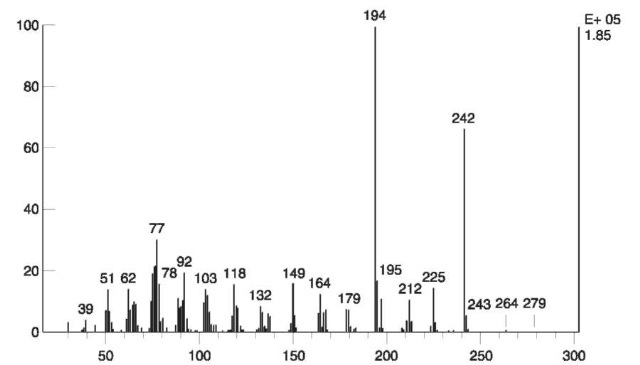
Figure 13 EI mass spectrum of N-methylpicramide.
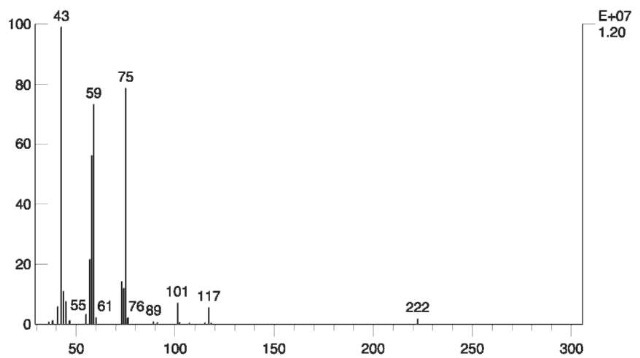
Figure 14 EI mass spectrum of TATP
Analysis procedures
A typical flow chart for postexplosion analysis includes sampling, visual examination, vapor analysis, extraction procedures and analysis by various methods. Extraction procedures include extraction by organic solvents for the organic explosives and aqueous extraction for the water-soluble compounds. Cleaning procedures may be carried out prior to the analysis, if required.
A flow chart of the generalized analysis of post-explosion is given in Fig. 15. The expert may choose a suitable debris procedure and an appropriate identification method according to the circumstances.
Sampling methods
Modern analytical methods can detect extremely small amounts of material, but no method, however sensitive, can detect an explosive if it is not present on the exhibit. Therefore, the collection of exhibits is a critical step in postexplosion analysis. Much work has been done to establish a methodology for the collection of exhibits according to their distribution around the bomb site. Unfortunately, no such methodology has been proved to be efficient and it seems that luck plays an important role in collecting the ‘right’ exhibit. Attempts to overcome this problem have been made by screening exhibits at the explosion scene, using kits based on color tests or ‘sniffing’ devices. Sniffing instruments are usually based on chemiluminescence detection (e.g. EGIS®) or ion mobility spectrometry (IMS) (e.g. IonScan®). The results of these preliminary tests are of indicative value only and cannot be regarded as an identification.
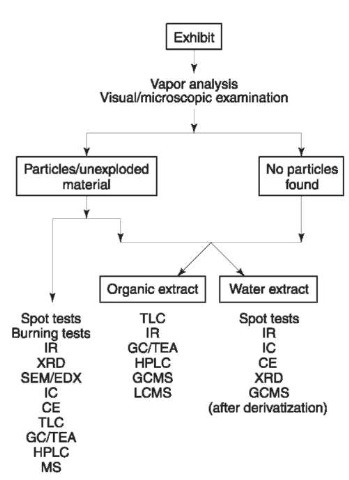
Figure 15 Flowchart of analysis of postexplosion debris
A major problem in postexplosion work is the potential contamination, either at the stage of collecting exhibits or during the subsequent laboratory analysis. Anyone collecting exhibits should also submit swabs of himself, taken before the sampling, to reveal the possibility of cross contamination. These swabs should be sent to the laboratory, considered as ‘blank samples’, and processed by the same procedures used for the exhibits.
Visual examination
It is highly recommended that the analysis of an exhibit begins with visual examination. As is usual in routine forensic work, nondestructive methods should be used first in order to extract information that may be lost after the use of destructive methods. Such information may include the morphological appearance of a particle which may connect a suspect to the scene if an identical particle is found in a suspect’s possession. The naked eye or a low-power stereoscope may be used for the physical separation of particles such as black powder, smokeless powder or material not consumed in the blast. Sieving may also be used for separation of these particles from debris.
Vapor analysis and adsorption on solid phase
Volatile explosives such as TATP or NG may be detected in vapors of an exhibit by direct headspace analysis. Adsorption of explosives from the vapor phase may be carried out at the scene (or in the laboratory) by passing the vapors through a suitable adsorbent material such as Amberlite XAD-7® or Tenax®. Explosives adsorbed on such resins may be eluted by a suitable solvent and then analyzed.
Fig. 16 shows mass chromatograms and total ion current (TIC) in which TATP was identified in vapors of soil, taken from a real-life case.
Organic extraction
Organic extraction is usually performed with acetone, which is the most commonly used solvent for explosive compounds. The solvent is then evaporated, under a stream of nitrogen rather than by heating, in order to minimize evaporation of volatile explosives. Acetone also dissolves nonexplosive materials from the debris such as oily compounds (e.g. hydrocarbons, fatty acids), plasticizers (e.g. phthalates) and some polar compounds. These materials may coelute with the explosives in chromatography and even change the Rt of the explosives. These coeluting compounds may also cause a significant decrease in instrumental performance. For example, contamination of the injection port, column and ion source in GC/MS results in decrease in sensitivity and resolution. To minimize extraction of oily compounds, ethanol/water mixtures rather than acetone may be used to swab exhibits.
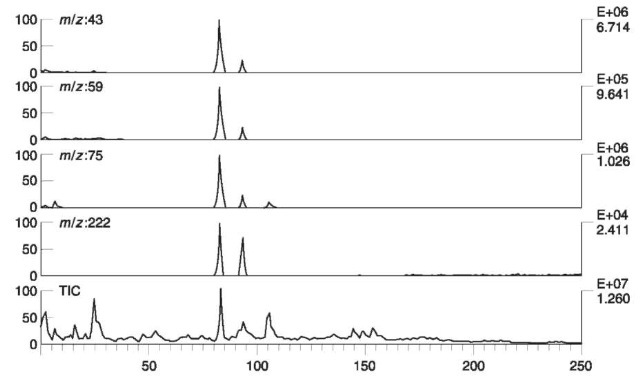
Figure 16 Total ion current (TIC) and mass chromatograms at m/z 43, 59, 75 and 222, of soil taken from a real-life case. Vapors of soil were adsorbed on active charcoal and eluted by CS2. TATP was identified in the peak emerging after 83 s.
Cleaning procedures In order to reduce the amounts of contaminants in the extract, cleaning procedures may be carried out prior to the analysis. They include liquid/liquid extraction, preparative TLC or HPLC, and solid phase extraction (SPE). SPE employs a suitable adsorbent packed in a column or in commercially-available cartridges. The extract is mounted on the adsorbent; starting with nonpolar eluting solvents (e.g. hexane), the hydrophobic compounds (e.g. hydrocarbons) are washed out first and the explosives are eluted later, when more polar solvents are used.
Analysis Analysis of a completely unknown compound often starts with screening tests (e.g. TLC, HPLC) followed by a suitable confirmation method (e.g. MS). In addition to serving as a screening method, GC/TEA, being highly specific, may also serve as a confirmation method. An advantage of using TEA detection rather than UV detection in postexplosion analysis is demonstrated in Fig. 17. A ‘real sample’ with traces of PETN was analyzed by HPLC with UV at 210 nm (upper trace) and by GC/TEA (lower trace). PETN could not be unequivocally identified in HPLC/ UV chromatogram whereas the chromatogram obtained by GC/TEA could be safely interpreted.
The forensic explosive laboratory at the Defence Evaluation and Research Agency (DERA) in the UK performs three consecutive GC/TEA analyses using three different columns. An identification of an explosive is concluded only when all three analyses are positive for the compound. GC/MS has been successfully used in the analysis of organic extracts of post explosion debris. Fig. 18 shows the TIC and mass chromatogram of the ion at m/z 46 where RDX was identified in the extract of postexplosion debris from a real-life case
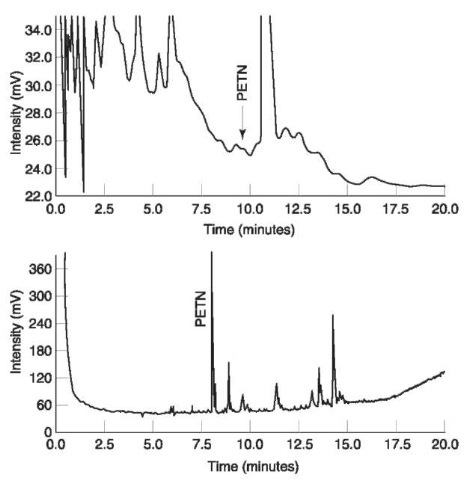
Figure 17 Upper trace:HPLC chromatogram with UV detection at 210nm of a ‘real sample’ with traces of PETN. Lower trace:chromatogram of the same sample analyzed by GC/TEA. Reprinted from Kolla P (1991) Trace analysis of explosives from complex mixtures with sample pretreatment and selective detection. Journal of Forensic Science 36: 1342, with permission from ASTM..
Some work has been carried out using negative ion mass spectrometry. IR is usually not suitable for postexplosion analysis due to the large amount of contaminants which interfere with the spectrum. The application of NMR to postexplosion extracts has had limited success, mainly due to its low sensitivity.
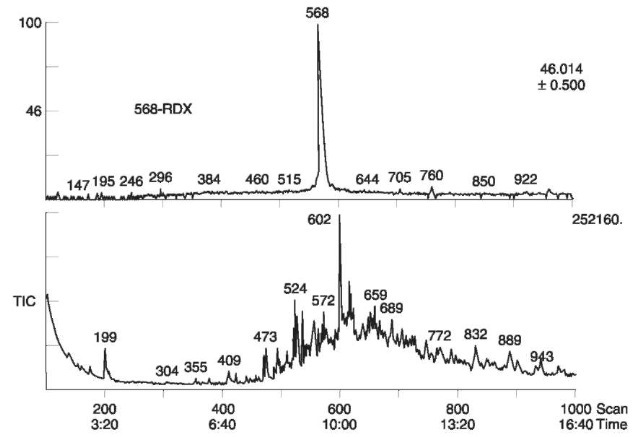
Figure 18 TIC and mass chromatogram at ion m/z 46, of postexplosion debris from real-life case. RDX was identified in the peak emerging after 568 s.
Aqueous extraction and analysis
Water is used to extract hydrophilic, water-soluble compounds. The dried extracts are then subjected to further analysis. Inorganic explosive-related anions may be detected by spot tests and confirmed by IC or CE. IR may also be used for the identification of these anions and other water-soluble compounds such as sugars. Unequivocal identification of some explosive-related inorganic anions may be carried out by GC/ MS. Nitrate, nitrite, thiocyanate and sulfide anions are derivatized by a suitable reagent such as penta-fluorobenzylbromide (PFBB), to produce volatile compounds which are easily analyzed by GC/MS. Figs 19 and 20 show EI mass spectra of the derivatiza-tion product of nitrate and thiocyanate, respectively. This method has been successfully applied to real-life casework.
4. Criteria for Identification
In forensic analysis where the results may affect the verdict in a court of law, a mistake must be avoided at all costs. Hence an identification of an explosive should be concluded only if strict criteria are met. It is generally accepted that unequivocal identification cannot be based on spot tests alone, as the results are presumptive and can at best indicate the possible presence of the chemical class to which the explosive belongs. The situation is more complex with chro-matographic methods. The conservative view has been that the identification of a single organic compound could not be based on chromatographic methods alone even if a combination of several such methods was used. Results from methods such as IR, MS or NMR are also required, because they reflect properties which are more directly related to the molecular structure of the analyte than chroma-tographic methods. This criterion has posed difficult problems in postexplosion analysis. The chromato-graphic results, which indicated the presence of an explosive, could not always be confirmed by such methods because of the extremely small amounts of unreacted explosives mixed with the large amounts of contaminants.
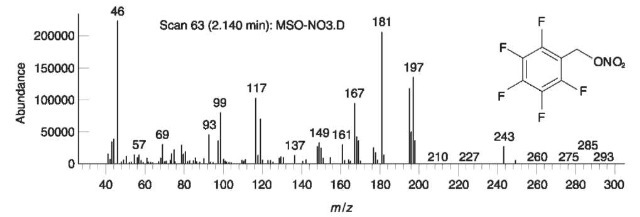
Figure 19 EI mass spectrum of the derivatization product of nitrate.
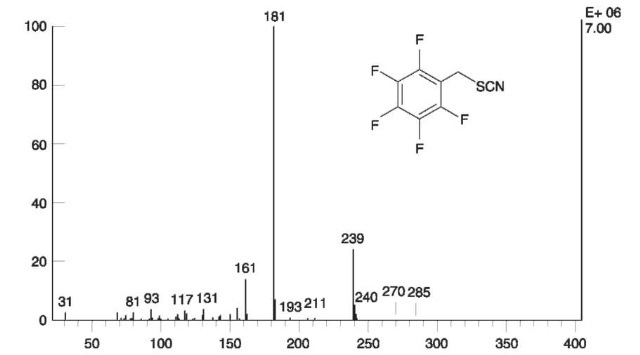
Figure 20 EI mass spectrum of the derivatization product of thiocyanate with PFBB.
This view is currently being re-examined by some forensic analysts. It can be argued that the introduction of highly specific detectors to chromatographic methods (mainly GC and LC) could change the criteria in such a way that a positive result in a chroma-tographic analysis could suffice for unequivocal identification of an analyte. The most striking example is the introduction of the TEA for GC and HPLC analysis of nitro-containing explosives. An indispensable prerequisite for a positive response of the TEA is the presence of nitro (or nitroso) group. Without their presence there would be no response. This enhanced specificity has led forensic analysts to regard chro-matographic retention data combined with a positive TEA response as sufficient evidence for the identification of nitro-containing explosives.
As can be seen in this chapter to obtain optimum results in the analysis of explosives in forensic laboratories the most modern and sophisticated instrumentation should be used. The human factor, however, remains the most important element in forensic science. It is the skill, experience and intelligent judgment of the chemist which determine the quality of the analytical results.
