Geographic distribution and map
Chikungunya fever has an epidemiological pattern with both sporadic cases and epidemics in west Africa, from Senegal to Cameroun, and in many other African countries (Democratic Republic of Congo, Nigeria, Angola, Uganda, Guinea, Malawi, Central African Republic, Burundi, and South Africa). Moreover, many epidemics occurred in Asia (Burma, Thailand, Cambodia, Vietnam, India, Sri Lanka, Timor, Indonesia, and the Philippines) in the 1960s and in the 1990s (Jain et al., 2008; Pialoux et al., 2007).
Major epidemics appear and disappear cyclically, usually with an inter-epidemic period ranging from 7 to 20 years. The huge outbreak that increased concern about CHIKV started in Kenya in 2004, where the seroprevalence rates reached 75% in Lamu island (Pialoux et al., 2007), before reaching the Comores, Seychelles, and Mauritius islands. The virus reached La Reunion island, a French overseas district, in March-April 2005, probably as a result of importation of cases among immigrants from the Comores. The outbreak had two phases: after some thousands of cases which occurred in March-April 2005, very few cases were reported during the austral winter, while the second epidemic peak arose in the initial months of 2006. For the first time, a substantial number of deaths (254) were attributed, directly or indirectly, to CHIKV. From late 2005 onwards, hospitals in some Indian states found themselves swamped with patients complaining of fever and joint pain, which turned out to be Chikungunya fever (Fusco et al., 2010). The World Health Organization Regional Office for South-East Asia has reported that 151 districts in nine states/ provinces of India have been affected by Chikungunya fever between February and October 2006 (Pialoux et al., 2007).
Several imported cases were reported in industrialized countries among travellers returning from endemic areas, mainly tourists and immigrants (Depoortere & Coulombier, 2006). In particular, many cases were detected in early 2006, when the outbreak involved the Indian Ocean islands. The Indian Ocean islands, India, and Malaysia are popular tourist destinations. According to the World Tourism Organization, an estimated 1 474 218 people travelled from Madagascar, Mauritius, Mayotte, Reunion, and the Seychelles to European countries in 2004 (Depoortere & Coulombier, 2006; Parola et al., 2006).
The European country with the highest number of imported cases was France, especially the south-eastern region of Provence-Alpes-Côte d’Azur, and Marseille in particular, home to a large Comorian community (Cordel et al., 2006; Hochedez et al., 2007). Other European countries that reported imported cases include Belgium, Bosnia, Czech Republic, Croatia,
Germany, Greece, Italy, Serbia, Spain, Switzerland, Norway, and the United Kingdom (Beltrame, A. 2007; Deporteere & Coulombier, 2006; Fusco, F.M. 2006; Pialoux et al., 2007; Taubitz et al., 2007). In 2006, CHIK fever cases have also been reported in traveller returning from known outbreak areas to Canada, the Caribbean (Martinique), and South America (French Guyana). During 2005-2006, 12 cases of CHIK fever were diagnosed serologically and virologically at CDC in travellers who arrived in the United States from areas known to be epidemic or endemic for CHIK fever, and 26 additional imported cases with onset in 2006 underscores the importance of recognizing such cases among travellers (CDC, 2006; CDC 2007).
Moreover, CHIKV gave rise in 2007 to the first autochthonous European outbreak in Italy, in the northern region of Emilia-Romagna (Rezza et al., 2007; Charrel et al., 2008). In June 2007, an Indian citizen returned to Italy after a visit to relatives in Kerala, India, developed 2 episodes of fever. During the second febrile episode, he visited his cousin in Castiglione di Cervia. The cousin had an onset of symptoms, with fever and arthralgia, on July 4. This sequence of events started the first Chikungunya fever outbreak in a temperate country, that lasted approximately 2 months with a total 247 cases of Chikungunya fever occurred in the region (217 laboratory-confirmed, 30 suspected) (Fusco et al., 2010). A unique sequence of events seems to have contributed to the establishment of local transmission in Emilia-Romagna: the high concentration of competent vectors A. albopictus in the area at the time of arrival of the index case, the presence of a sufficient human population density and the temporal overlapping of arthropod activity (seasonal syncronicity) (Charrel et al., 2008; Rezza et al., 2007).
During 2008, cases of Chikungunya fever have been reported from many countries in Asia other than India, as well as active epidemics from Singapore, Sri Lanka, and Malaysia (Leo et al., 2009).
Since 2006, the Regional Office of the French Institute For Public Health Surveillance in the Indian Ocean has conducted epidemiological and biological surveillance for CHIKV infection. During the period December 2006-July 2009, no confirmed case was detected on Reunion Island and Mayotte, but new outbreaks were reported in Madacascar. After few years of relative dormancy in Réunion Island, in August 2009, a cluster of cases was identified on the western coast of Réunion Island (D’Ortenzio et al., 2009) and, subsequently, an outbreak of CHIKV infection was described on Réunion Island in 2010 (D’Ortenzio et al., 2011). Moreover, recent publications described cases of Chikungunya fever in tourist returning from Maldives, confirming the circulation of the virus by the end of 2009 (Pfeffer et al., 2010; Receveur et al., 2010)
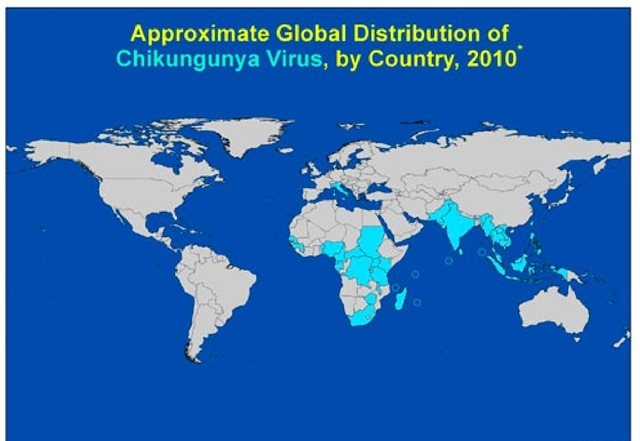
These episodes have refreshed the concerns about the possibility of renewed autochthonous transmission in Mediterranean countries and highlight the need for surveillance in countries where emerging infections may be introduced by returning travellers. Travellers can serve as sentinel population providing information regarding the emergence or re-emergence of an infectious pathogen in a source region. Travellers can thus act as carriers who inadvertently ferry pathogens that can be used to map the location, dynamics and movement of pathogenic strains (Pistone et al, 2009). Thus, with the increase in intercontinental travel, travellers can provide insights into the level of the risk of transmission of infections in other geographical regions. The geographic range of CHIKV is mainly in Africa and Asia (Fig. 1)
Fig. 1. Geographical distribution of CHIKV shown in the most recent map coming from the CDC’s Traveler’s Health website (http://wwwn.cdc.gov/ travel/default.aspx).
Phylogenesis
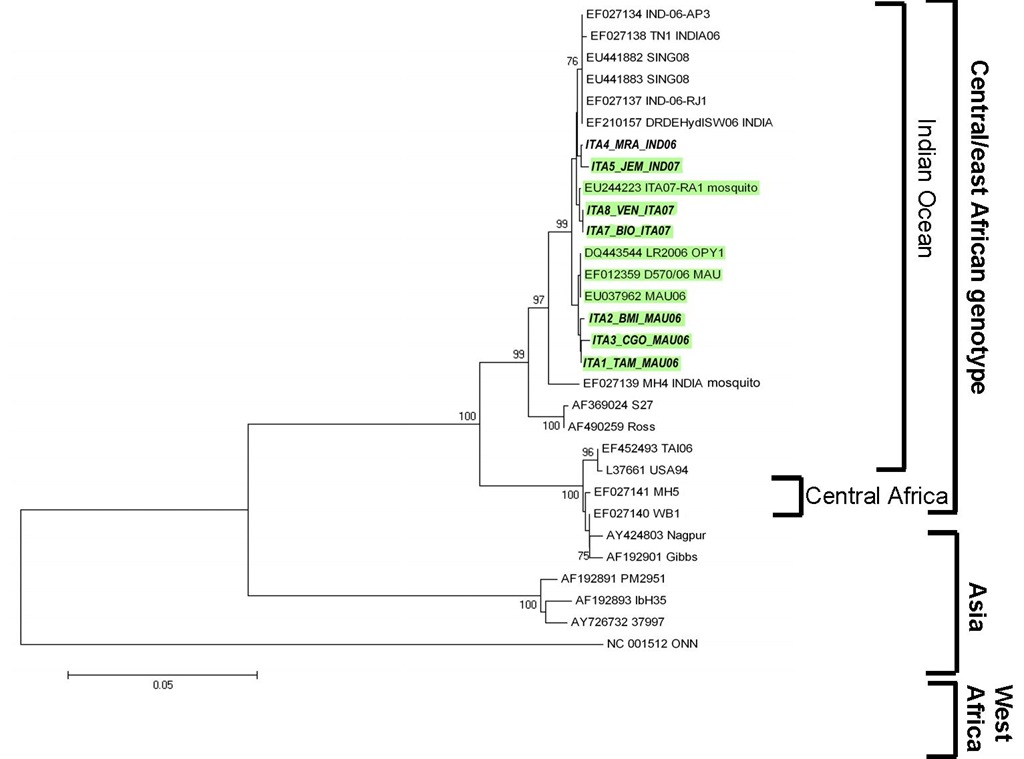
Three lineages of CHIKV, with distinct genotypic and antigenic characteristics, have been identified. Isolates that caused the 2004-06 Indian Ocean outbreak form a distinct cluster within the large eastern and central Africa phylogenetic group, in addition to the Asian and west African phylogenetic groups (Powers et al., 2000; Schuffenecker et al., 2006). Phylogenetic analysis of CHIKV strains circulating in A. Albopicus-humans transmission cycles, obtained during outbreaks, have identified the independent acquisition of a common mutation in E1 glycoprotein (E1gp), namely A226V, in strains isolated from different geographic regions (Schuffenecker et al., 2006; de Lambellerie et al., 2008). This mutation, together with M269V, D284E mutations of E1 CHIKV glycoprotein have been described as molecular signatures of the Indian Ocean outbreak (Arankalle et al., 2007; Tsetsarkin et al., 2007; Vazeille at al., 2007). In particular, the A226V mutation, which was absent in the strains isolated during the initial phases of the outbreak in Réunion, appeared in >90% of the isolates after Dicember 2005. This change could be related to virus adaptation to the mosquito vector species. Together with the lack of herd immunity, this might explain the abrupt and escalating nature of the Reunion outbreak. Has been clearly demonstrated that the A226V mutation is able to increase viral fitness in the Aedes albopictus vector (Tsetsarkin et al., 2007; Vazeille et al., 2007), that, in turn, may expand the potential for CHIKV to diffuse to the Americas and Europe, due to the widespread distribution of this vector, in particular in Italy (Knudsen, 1995). In a previous paper we characterized 7 viral isolates (5 imported and 2 autochthonous cases), with respect to the molecular signatures of the Indian Ocean Outbreak in E1, particularly the A226V mutation. Imported cases included 3 returning from Mauritius in 2006 and 2 returning from India in 2006 and 2007, respectively; the autochthonous cases occurred during the 2007 Italian outbreak (Bordi et al., 2008). CHIKV sequences of a 1013 bp fragment of E1 gene (nucleotide positions 10145-11158, respect to the reference strain S27) have been analyzed (Fig.2).
All 7 isolates carried the M269V and D284E Indian Ocean signatures while the A226V mutation was present in all the isolates imported from Mauritius, in the autochthonous cases from the Italian outbreak and in the isolate imported from India in 2007, but was absent in the case imported from India in 2006.
Our findings indicated that, during 2006 and 2007, multiple strains have been imported to Italy from countries where explosive Chikungunya outbreaks were ongoing. All the strains isolated in Italy, both imported and autochthonous, displayed two molecular signatures of the Indian Ocean outbreak (M269V and D284E). Concerning the A226V mutation, this was present in all imported and autochthonous cases, with the exception of the isolate imported from the Indian subcontinent in 2006. The absence of this mutation in the isolate imported in 2006 from India was in agreement with published data (Arankalle et al., 2007), and with available GenBank sequence data, indicating that the virus strains circulating in India in 2006 lacked this mutation.
Fig. 2. Phylogenetic tree of CHIKV strains performed on partial E1 gene CHIKV sequences of a 1013 bp fragment of E1 gene (nucleotide positions 10145-11158, respect to the reference strain S27) have been analyzed. The strains isolated from human cases in Italy are in bold (Bordi et al., Clin Infect Dis, 2008) \
The presence of A226V in the isolate imported from India in July 2007 and in the isolates from the 2007 Italian outbreak (originating from a case imported from India) supports the view that the virus envelope sequence of strains from India changed over time, acquiring after 2006 the E1 mutation associated with enhanced fitness in Aedes albopictus. So it appears that the acquisition and fixation of the A226V mutation may be a common pathway of chikungunya explosion in epidemic areas, in a parallel interplay with the mosquito vector dynamics. Noteworthy, the outbreak in Singapore, where the A226V mutation was absent, has been rapidly controlled.
Immune-pathogenesis
Given the expanding geographic range of CHIKV and its potential to rapidly cause large scale epidemics, it has become important to understand the immune and pathogenic mechanisms active during CHIKV infections in order to guide the development of targeted and effective control and treatment strategies.
In a review the possible interactions of the immune system with the different stages of the CHIKV life cycle have been discussed (Kam et al., 2009). The first encounter of CHIKV with human host is intradermal inoculation by the mosquito: replication of the virus starts at the site of inoculation. Different resident cell types are present in this location, including keratinocytes, dermal dendritic cells (DCs), Langerhans cells (LCs), and dermal macrophages, cells involved in the innate immune response.
The innate immune response is the first barrier against viruses, being able to inhibit viral replication through cytolytic and non-cytolytic mechanisms. IFN system plays an important role in limiting virus spread at an early stage of infection. In vitro growth of all tested alphaviruses can be greatly suppressed by the antiviral effects of Interferon-a/ β (IFN-α/β) when it is added to cells prior to infection, and, more specifically, CHIKV replication is significantly influenced by type I and II IFNs (Courderc et al., 2008; Schilte et al., 2010; Sourisseau et al., 2007). The finding that aberrant Type I interferon signalling in mice led to severe forms of CHIKF (Couderc et al., 2008) further highlighted important roles cytokines play in the pathology of CHIKV infection. Moreover, in a very recent study Wauquier and colleague demonstrated that CHIKV infection in humans elicit strong innate immunity involving the production of numerous proinflammatory mediators. Interestingly, high levels of Interferon IFN-α were consistently found. Production of interleukin (IL) 4, IL-10, and IFN-γ suggested the engagement of the adaptive immunity. This was confirmed by flow cytometry of circulating T lymphocytes that showed a CD8+ T lymphocyte response in the early stages of the disease, and a CD4+ T lymphocyte mediated response in the later stages (Wauquier et al., 2011).
It was already known that skin cell fibroblasts were susceptible to CHIKV infection (Sourisseau et al.,2007); recently has also been demonstrated that CHIKV antigens could be detected in vivo in the monocytes of acutely infected patients (Her et al, 2010). CHIKV interactions with monocytes, and with other blood leukocytes, induced a robust and rapid innate immune response with the production of specific chemokines and cytokines. In particular, high levels of IFN-a were rapidly produced after CHIKV incubation with monocytes. The identification of monocytes during the early phase of CHIKV infection in vivo is significant as infected monocyte/macrophage cells have been detected in the synovial tissues of chronically CHIKV-infected patients, and these cells may behave as the vehicles for virus dissemination. This may explain the persistence of joint symptoms despite the short duration of viraemia (Her et al., 2010).
Since the A226V mutation has been associated with enhanced replication and fitness of CHIKV in A. albopictus vector and has also been shown to modulate cholesterol requirement for infection of insect cells (Tsetsarkin et al., 2007), in a recent paper we investigated the possible involvement of A226V mutation in enhancing human pathogenesis in non vector hosts, by testing the replication competence in primate cell cultures of two isolates, differing for the presence or absence of this mutation (Bordi et al., 2011). We observed that the presence of A226V mutation did not influence the replication kinetics on primate cells. Moreover, the time course of appearance of cytopathic effect (CPE) and of cells immunostained with CHIKV-specific antiserum, was very similar for both the isolates, as well as the shape of the virus-positive multicellular foci, thus suggesting a similar mechanism of spread of the virus in the infected cell cultures.
In addition, we considered the possibility that the A226V mutation could be associated with partial resistance to the inhibitory action of IFN-a in classical experiments of inhibition of virus replication. Surprisingly, the A226V-carrying strain was more susceptible to the antiviral action of recombinant IFN-a. (Fig.3)
Fig. 3. Dose-dependent reduction of viral CPE by recombinant IFN-a.
In vitro experiments of inhibition of virus replication by recombinant IFN-a on Vero E6 cells showing a dose-dependent reduction of CPE for both isolates: the A226V, carrying isolate and the wt (Bordi et al., New Microbiol, 2011).
Overall, our result did not support the concept that A226V mutation confers a replicative advantage in primate cell cultures, neither supported the possibility that partial resistance to the inhibitory action of IFN-a could account for the explosive spread of the mutated strain in the human population in the countries where this mutation had occurred. However, the possibility that the interplay between the virus and the innate defence system may act at different levels of the virus/host interaction is to be taken into consideration, by exploring, for instance, other steps of the IFN response activation.
At the moment, understanding CHIKV immuno-biology is still in its infancy and there is a long way to go before answers related to the interaction between virus and host immunity will be obtained. These will certainly be important in designing novel antiviral control strategies against the spread of CHIKV infection.