Treatment
The drug of choice for the treatment of E. chaffeensis infection is the tetracyclines (particularly doxycycline) and their derivatives. Generally, between 1 and 3 days after a patient with HME commences treatment with doxycycline, the patient becomes afebrile (Olano & Walker, 2002). However, treatment may continue for up to 10 days or at least 3 days after the patient becomes afebrile (Chapman et al., 2006). Clinical experience and in-vitro susceptibility testing of E. chaffeensis to some classes of antibiotics have revealed that fluoroquinolones, penicillins, aminoglycosides, macrolides and cotrimoxazole are not effective therapeutics (Dumler et al., 1993; Brouqui et al., 1994; Brouqui & Raoult, 1994; McBride & Walker, 2010).
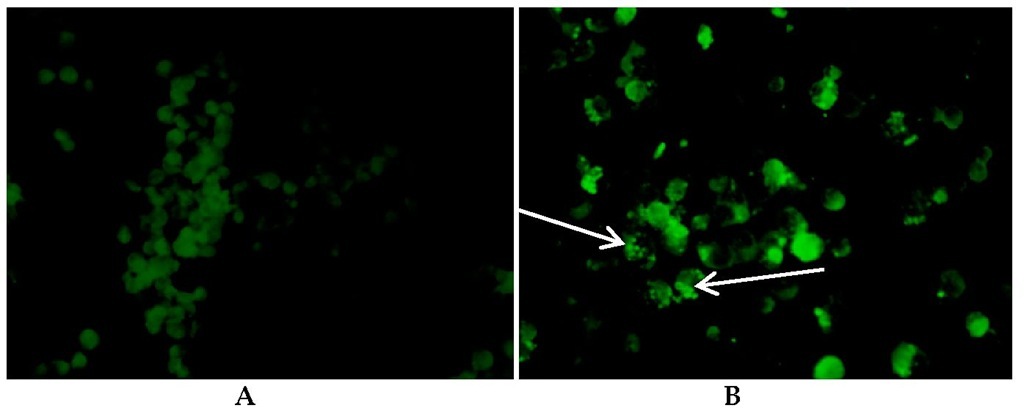
Fig. 3. Reactions of patient serum with Ehrlichia chaffeensis antigen and staining with Fluorescein isothiocynate (FITC)-labelled goat, IgG anti-human antibody (X40 magnification). A: Negative IFA slide, no inclusion bodies in cells. B: Positive IFA slide with morulae in monocytes (arrows).
Rickettsiosis
The Genus Rickettsia
Rickettsial organisms are Gram-negative bacteria belonging to the order Rickettsiales, Family Rickettsiaceae and the Genus Rickettsia. They are strict intracellular parasites that are transmitted by arthropods including fleas, lice, mites and ticks (Kelly et al., 1992). These organisms are typically short rods (coccobacilli) measuring about 0.8-2^m in length and 0.3-0^m in diameter. They exhibit most of the biochemical and morphological characteristics of the Gram-negative cell (Gimenez, 1964; La Scola & Raoult, 1997). Based on antigenic characteristics, species of the genus Rickettsia have been divided into three groups; namely the typhus group (TG), the spotted fever group (SFG) and the transitional group (TRG). The TG has two members (R. prowazekii and R. typhi), which are mainly transmitted by lice and fleas, respectively (Raoult & Roux, 1997). The largest of the antigenic groups is the SFG that is made up of the tick-transmitted pathogens (except R. bellii and R. canadensis) (Parola et al., 2005). It has been grouped into several genogroups based on the 16S rRNA, the gltA, ompA, ompB and sca2 sequences. These groups include the R. rickettsii group, the R. massiliae group and R. helvetica group. The transitional group includes R. akari, R. australis, and R. felis. There are many ancestral organisms including R. canadensis and R. bellii (Parola et al., 2005), as well as numerous rickettsiae in herbivorous insects, and other hosts (leaches and amoeba). Also in the family Rickettsiaceae is Orientia tsutsugamushi, which is transmitted by Leptotrombidium deliense (Tamura et al., 1995).
Rickettsial organisms are of worldwide occurrence, although species/vector differences may exist along various geographical lines. In Africa, several species have been reported. These include R. conorii Malish strain, the cause of Mediterranean spotted fever or "boutonneuse fever". It was first documented in Tunis (Conor & Bruch, 1910), and today the disease continues to be reported in Tunisia (Romdhane et al., 2009; Sfar et al., 2009) and South Africa. The infection has the characteristic of a papular rash, in addition to an eschar at the site of the tick bite (Anton et al., 2003). The pathogen is transmitted by R. sanguineus ticks, and is considered an urban disease (Font & Segura, 1983). Human infections with another strain of R. conorii (Israeli spotted fever strain) have been recently documented in Tunisia (Znazen et al., 2011). R. conorii Astrakhan strain is the cause of Astrakhan fever first detected in Astrakhan, Russia in the 1970s and transmitted by R. sanguineus and R. pumilio ticks (Parola et al., 2005). The Astrakhan strain has also been isolated from a patient in Chad (Fournier et al., 2003). R. sibirica mongolitimonae strain was identified in Hyalomma truncatum ticks in Niger in 2001 (Parola et al., 2001) and the first human case in Africa was documented in South Africa (Pretorius & Birtles, 2004). Other cases have been reported to have been acquired in Algeria (Fournier et al., 2005) and Egypt (Socolovschi et al., 2010). Another species, R. aeschlimannii, which was first isolated in H. marginatum ticks in Morocco (Beati et al., 1997) and later detected in H. marginatum rufipes in Mali and Niger, have been known to cause infections in tourists returning from Morocco and South Africa (Parola et al., 2001). R. massiliae, first isolated from R. sanguineus ticks in Marseille, France (Parola et al., 2005) was detected in R. muhsame, R. lunalatus and R. sulcatus from Central African Republic (Dupont et al., 1994) and in R. muhsame ticks collected from cattle in Mali 2001) and Ivory Coast (Berrelha et al., 2009). Rickettsia felis is a recently identified pathogen which was first detected in Ctenocephalides felis fleas (Bouyer et al., 2001). In Africa, the agent has been reported in Ivory Coast (Berrelha et al., 2009) and Senegal (Socolovsch et al., 2010), and human infections have been reported in Kenya (Richards et al., 2010). Rickettsia africae, the etiologic agent of African tick bite fever appears to be the most prevalent rickettsiosis in Africa. The disease was first reported in Mozambique and South Africa (McQuiston et al., 2004). The first isolate (R. africae strain ESF-5), was recovered from A. variegatum ticks in Ethiopia although it was only characterized as R. africae later (Roux et al., 1996). The agent was later isolated in A. hebraeum ticks in Zimbabwe in 1990, and in 1992, the first isolate from a patient was obtained (Kelly et al., 1991; Kelly et al., 1994). The pathogen has been detected in many other African countries including Senegal (Mediannikov et al., 2010), Ethiopia (Stephany et al., 2009) and Cameroon (Ndip et al., 2004a; Ndip et al., 2004b). In the following, we give a synopsis of our current knowledge of African tick bite fever in Cameroon.
Causative organism
R. africae, a member of the SFG is the only species that has been detected in Cameroon. The organism which measures about 0.4 μ^ι by 1.0 μ^ι, has an outer slime layer and a trilaminar cell wall which contains immunogenic lipopolysaccharide antigens responsible for cross-reactivity with the other SFG rickettsiae (Hechemy et al., 1989; Kelly et al., 1996). Like other Gram-negative organisms, rickettsiae have outer membrane proteins (dubbed OmpA and OmpB) present as species-specific antigens (Fournier et al., 1998; Roux & Raoult, 2000). The organism lives freely in the cytoplasm and usually infects endothelial cells. According to phylogenetic studies, this rickettsial species, which belongs to the R. rickettsii group is closely related to R. parkeri in North America and R. sibirica in northeast Asia (Parola et al., 2005).
Tick vectors and reservoirs
R. africae is a tick-borne pathogen, and ticks serve both as vectors and reservoirs. The pathogen is maintained in the tick through trans-stadial and trans-ovarial transmission, and this situation indicates the potential for transmission to humans by all stages (larvae, nymphs, and adults) of the feeding ticks. Ixodid ticks (hard ticks) of the genus Amblyomma have been recognized as the vectors (Kelly, 2001). In Cameroon, A. variegatum (Figure 1b) has been identified as the potential vector with about 75% of ticks (male and female) collected from cattle found to be infected with R. africae (Ndip et al., 2004b). Reports from other studies have indicated that R. africae infection in Amblyomma ticks frequently has a high prevalence (up to 100%) reported in ticks collected in some disease-endemic countries (Dupont et al., 1994; Parola et al., 2001). Like any other tick borne disease, the ecological characteristics of the vector influence the epidemiology of the disease. The ticks are usually found all year round, but they peak during and after the rainy season when humidity is very high (Walker et al., 2003). Ambylomma are predominantly cattle ticks, and infestation of cattle can be very high (Kelly & Mason, 1991). A. variegatum, commonly found in central and west Africa typically enjoys a wide variety of different habitats although they have a preference for semi-arid and humid areas with tall grass, trees, and/or bush cover. These ticks usually quest on vegetation and would usually attack legs although they may crawl to other areas such as groin and perineum where they attach (Jensenius et al., 2003).
Clinical presentation
Since the description of ATBF in 1992, most of the knowledge available regarding the disease has been documented in travelers who become infected with R. africae during travel in Africa. After inoculation from a tick bite, the bacteria invade the vascular endothelial system causing a focal or disseminated vasculitis. Endothelial cells of small blood vessels become infected leading to the destruction of the endothelial cells (Toutous-Trellu et al., 2003) of the host where they have multiplied and eventually injured the host cells, leading to the disease symptoms. Multiple eschars typical of ATBF develop at the sites of tick bite, and following an incubation period between 5 and 7 days (up to two weeks in some cases), after the tick bite a febrile illness develops (Raoult et al., 2001). In most cases, symptoms of ATBF are usually mild and include headaches, nausea, chills, myalgia, lymphadenopathy and prominent neck ache (Jensenius et al., 2003; Raoult et al., 2001). Although there have been some controversies over the differences in the clinical presentations of African tick bite fever, our study of acutely ill patients in Cameroon revealed that the some individuals may manifest severe symptoms while in others the symptoms are mild. However, symptoms reported include fever >38°C (100%), headache (71%), myalgia (71%), arthralgia (57%), rash (15%) and pulmonary signs (28%).
Epidemiology
ATBF has been recognized as an emerging problem in sub-Saharan Africa, especially for international travelers to rural areas (Jensenius et al., 2003). Most of the victims reported are tourists who visit game reserves or participate in outdoor activities such as running, trekking and hiking in forested areas, usually inhabited by Amblyomma ticks. The patients acquire the disease in rural Africa, but most often symptoms manifest only after they have returned to their various countries in Europe and America. The first report suggesting that rickettsiosis could be prevalent in Cameroon was published in 1968 (Maurice et al., 1968). The report based on a serologic survey that used an unreliable technique demonstrated rickettsial antibodies in cattle and humans in the northern region of Cameroon and in other animals in the south of the country ( Maurice et al., 1968; Le et al., 1977). Efforts to determine the epidemiology and ecology later re-emerged in 2004 when anti-rickettsial IgM antibodies were detected in some Cameroonian patients along the coastal region of Cameroon (Ndip et al., 2004a). These results were further confirmed by detection of R. africae DNA in about 6% of acutely ill febrile patients (Ndip et al., 2004b). Human infections or the agent has been detected in all regions of southern Cameroon where epidemiologic investigations have been made (Figure 3). According to these studies, age appeared to be a risk factor of acquiring the disease, and it is suggested that activities such as game hunting usually constitutes a risk factor (Ndip et al., 2011). Other activities which could predispose to infection include cattle rearing and exposure to tick habitats.
Cameroon is a sub-saharan tropical country with a vast equatorial forest providing a good habitat for ticks (especially A. variegatum ticks). Individuals residing in lowland rainforest habitats have a higher risk of acquiring ATBF probably because these habitats are ideal for A. variegatum ticks because of their moderate canopy cover, providing microclimates favoring tick survival (Ndip et al., 2011). Although ATBF has been shown to be prevalent in the southern part of Cameroon (Figure 2), the actual epidemiology of the disease through wider disease surveillance needs to be documented.
Diagnosis
Diagnosis of ATBF can be achieved by either serological analysis of acute and convalescent serum samples or molecular detection of the DNA of the bacterium by real-time or conventional PCR. Target genes that have been utilized include the rickettsial gltA and ompA genes. For serological diagnosis, the indirect immunofluorescent test has been used in conjunction with western blot assay to detect antibodies reactive with whole cells or specific proteins of cell lysates of R. africae. However, these tests are not very reliable in distinguishing species because cross-reactivity may be observed among the SFG rickettsiae. However, some authors have proposed that a fourfold or greater titer for R. africae compared to other species is confirmatory (Raoult et al., 2001; Ndip et al., 2004a). The western immunoblot assay can also be used to detect antibodies against species-specific OmpA and OmpB proteins.
Treatment
The drug of choice for the treatment of ATBF is doxycycline (100 mg twice daily) for 3-7 days. In-vitro studies also indicate that R. africae is susceptible to tetracyclines, fluoroquinolones, some macrolides and chloramphenicol (Rolain et al., 1998). Mild cases of ATBF have also been shown to recover naturally (Jensenius et al., 1999).
Prevention of ehrlichiosis and rickettsiosis
Studies in Cameroon indicate that one risk factor for contracting E. chaffeensis infection and ATBF appears to be exposure to potential tick vectors. Many reports involving acquisition of rickettsial diseases have also indicated that exposure to ticks during safari tours and visit to parks constitute an important risk factor. Therefore, an important method of preventing ehrlichiosis and rickettsiosis is by reducing contact with infected ticks. Personal protective measures are quite important, including wearing light colored clothes when walking in tick infested areas, using insect repellents and examination of clothing after a visit to a tick infested area, and prompt removal of attached tick can all reduce the risk of infection. Companion animals and other domesticated animals should be taken care of and tick infestation controlled.
Conclusions
These data emphasize the importance of ehrlichiosis and ATBF as prevalent diseases in an indigenous Cameroonian population. Although these diseases present as febrile illnesses, they are rarely considered when evaluating patients with acute, undifferentiated febrile illnesses. This situation can be attributed in part to lack of adequate knowledge of the epidemiology and ecology of the disease to prompt diagnosis; unavailability of specific laboratory tests, equipment, and expertise and also the limited economic resources. Sharing new knowledge on these diseases and techniques to facilitate diagnosis are important factors that can change the types and frequencies of diseases diagnosed in febrile patients and necessitate surveillance for these diseases. Future efforts will attempt to address other issues requiring investigations such as the full description of the clinical spectrum of these diseases in African patients and risk factors for severe illness.