Introduction
Human ehrlichioses and rickettsioses are important arthropod borne infectious diseases which are transmitted by ticks, mites, lice and fleas. Infections result in mild to fatal outcomes, with clinical presentations that resemble other tropical infectious diseases such as malaria making clinical diagnosis difficult. Despite recognition as important causes of life-threatening diseases in the United States, the geographic distribution of these diseases worldwide remains undefined due to their recent emergence, challenges in diagnosis and lack of comprehensive epidemiological studies needed to determine incidence in developing countries. Recently, the transfer of technological developments to other parts of the world especially developing countries has encouraged basic epidemiological inquiry and generated scientific interest in understanding the epidemiology of these tick borne diseases and their role as causes of undifferentiated febrile illnesses. In this topic, we review the current knowledge of human monocytotropic ehrlichiosis (HME) and spotted fever rickettsiosis (African tick bite fever) in Cameroon.
Ehrlichiosis
Etiologic agents
Ehrlichioses are diseases caused by small (approximately 0.4-1.5 pm diameter) Gram negative, obligately intracellular bacteria belonging to the genus Ehrlichia of the family Anaplasmataceae, Order Rickettsiales and the alpha sub-division Proteobacteria (Dumler et al., 2001). Although they have a characteristic Gram negative cell wall structure, they lack the necessary enzymes to synthesize cell membrane components such as lipopolysaccharide and peptidoglycan (Lin & Rikihisa, 2003). As intracellular pathogens, Ehrlichia reside in cytoplasmic membrane-bound vacuoles inside host cells (granulocytes or monocytes) forming microcolonies called morulae, derived from the Latin word "morus" for mulberry (Popov et al., 1995; Paddock et al., 1997; Ismail et al., 2010). These morulae (ranging in size from 1.0 to 6.0 μηι in diameter) may contain 1 to >40 organisms of uniform or mixed cell types (Popov et al., 1995; Rikihisa, 1999).
Organisms in the family Anaplasmataceae were first described in 1910 when Theiler described Anaplasma marginale, the etiologic agent of an economically important and severe disease of cattle (Mahan, 1995). This discovery was followed shortly thereafter by the description of E. ruminantium (formerly Cowdria ruminantium) by Cowdry in 1925; E. canis by Donatien and Lestoquard in 1935; and A. phagocytophilum (formerly E. phagocytophila) by Gordon in 1940. Hence, the genus Ehrlichia was established in 1945 in honour of the German microbiologist Paul Ehrlich (Uilenberg, 1983).
Ehrlichia species cause significant diseases in their natural hosts (livestock and companion animals) and emerging zoonoses in humans (McBride & Walker, 2010). The first human ehrlichial infection (sennetsu fever) was reported in 1953 ( Rapmund, 1984; Dumler et al., 2007). Sennetsu fever, caused by Neorickettsia sennetsu, was identified in Japan and Malaysia (Dumler et al., 2001; Dumler et al., 2007). However, recent phylogenetic reclassifications based on molecular analysis revealed that E. sennetsu is not a member of the Ehrlichia genus (Dumler et al., 2001). Presently, the genus Ehrlichia consists of five recognized species including E. canis, E. chaffeensis, E. ewingii, E. muris, and E. ruminantium, all of which are at least 97.7% similar in 16S rRNA gene sequence (Perez et al., 1996; Paddock et al., 1997; Dumler et al., 2001; Perez et al., 2006).
Ehrlichiae have relatively small genomes (0.8-1.5 Mb) with low G+C content and a high proportion of non-coding sequences but can synthesize all nucleotides, vitamins and cofactors (Dunning et al., 2006). They also have small subsets of genes associated with host-pathogen interactions (Ismail et al., 2010). E. chaffeensis have immunodominant outer membrane proteins (OMP-1/MSP2/P28) (Ohashi et al., 1998; Yu et al., 2000; Huang et al., 2008), and in infected macrophages ehrlichiae express the p28-Omp 19 and 20 genes as dominant protein products (Ganta et al., 2009; Peddireddi et al., 2009). Ehrlichiae also express several targets of the humoral immune response including tandem repeat and ankyrin repeat containing proteins (Yu et al., 1997; Sumner et al., 1999; McBride et al., 2003; McBride et al., 2007). E. chaffeensis, a human pathogen that was first recognised in the United States in 1986 and isolated in 1991 ( Maeda et al., 1987; Dawson et al., 1991) is the cause of human monocytotropic ehrlichiosis (HME) (Anderson et al., 1992), a moderate to severe disease with a case fatality rate of 3% (Fishbein et al., 1994; McBride & Walker, 2010). E. chaffeensis is an obligately intracellular bacterium that primarily infects mononuclear leukocytes and replicates by binary fission. E. chaffeensis morulae can be detected in peripheral blood smears obtained from infected patients when observed with a light microscope (Rikihisa, 1991). When tissues (including clinical samples), mononuclear leucocytes or cell lines of mammalian origin infected with E. chaffeensis are viewed by electron microscopy, two distinct morphologic cell types are identified: a predominantly coccoid form which has a centrally condensed nucleoid DNA and ribosomes (dense-cored cells) measuring between 0.4 and 0.6 μ^ι in diameter and reticulate or the coccobacillary form, which measures about 0.4 to 0.6 μ^ι by 0.7 to 1.9 μ^ι (Paddock et al., 1995; Popov et al., 1997).
Vectors and reservoirs
Investigative studies following the discovery of E. chaffeensis in the late 1980s revealed that the agent is transmitted to humans by the tick Amblyomma americanum, commonly referred to as the lone star tick which has a limited geographic distribution to the United States (Anderson et al., 1993). Molecular analysis (PCR) has demonstrated E. chaffeensis DNA in adult A. americanum ticks collected from different states. The increased recognition of E. chaffeensis as an emerging problem has evoked renewed interest in this and other tick borne diseases, and this has stimulated epidemiologic investigations of this pathogen and its vector in other regions where the tick A. americanum is not found. Results not only indicate that E. chaffeensis has a wider distribution than the United States (Ndip et al., 2010), but also indicates that the pathogen exists outside of the known range of A. americanum and is harbored by other tick species. These tick species include Ixodes pacificus in California (Kramer et al., 1999), Dermacentor variabilis in Missouri (Roland et al., 1998), Ixodes ricinus in Russia (Alekseev et al., 2001), Amblyomma testudinarium in China, (Cao et al., 2000), Haemaphysalis longicornis (Lee et al., 2003), and Ixodes persulcatus (Kim et al., 2003) in Korea.
Fig. 1. a) Rhipicephalus sanguineus (brown dog tick) and b) Male Amblyomma variegatum tick
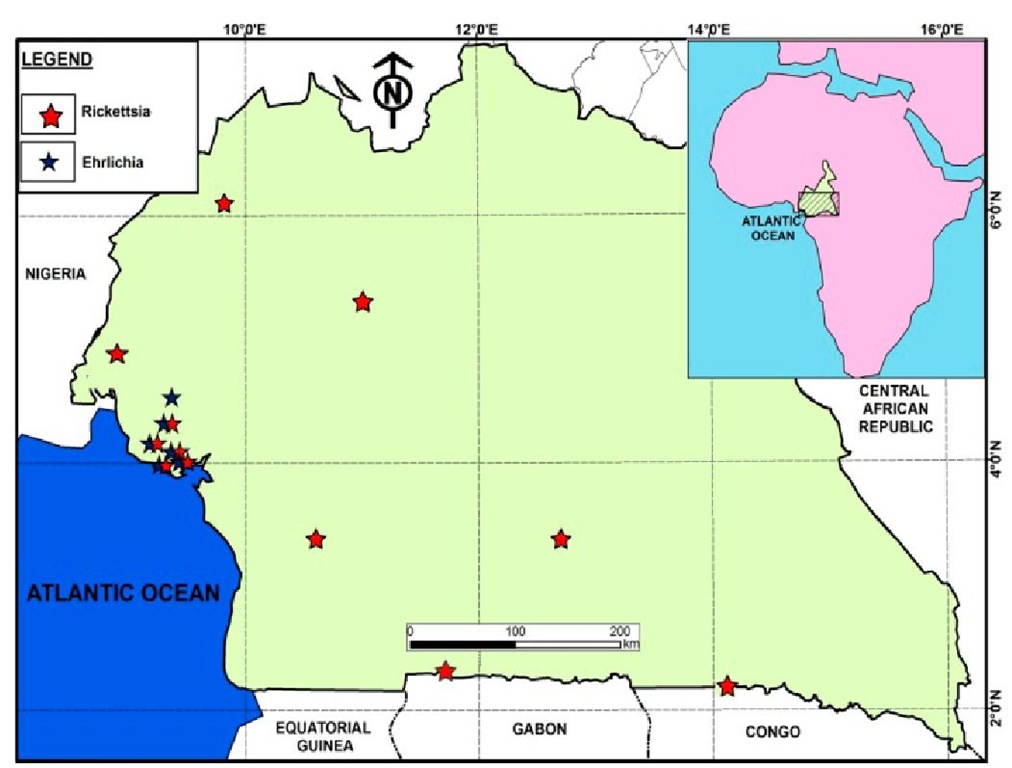
Studies carried out by Ndip and colleagues in Cameroon identified Ehrlichia chaffeensis in Rhipicephalus sanguineus ticks. R. sanguineus, commonly known as the brown dog tick (Figure 1a) is a species that infests canids worldwide. In one study in Limbe, Cameroon, a very high prevalence of E. chaffeensis was detected in R. sanguineus ticks infesting dogs inhabiting one kennel (Ndip et al., 2010). E. chaffeensis DNA was detected in 33 (56%) of 63 R. sanguineus ticks collected from five dogs as opposed to 4 (6%) ticks infected with E. canis. Furthermore, co-infection with more than one pathogen was not uncommon. The E. chaffeensis strain circulating in Cameroon is similar to the North American strain AF403710 based on the analysis of the 378 bp fragment of the disulphide bond formation (Dsb) protein gene (Ndip et al., 2010). Earlier reports revealed E. canis, E. chaffeensis, and E. ewingii in R. sanguineus ticks collected from 51 dogs from different localities in Cameroon (Figure 2), suggesting that dogs could be a reservoir for E. chaffeensis and that R. sanguineus is the probable vector (Ndip et al., 2007).
In the United States, the white-tailed deer (Odocoileus virginianus) has been recognised as the primary natural reservoir of E. chaffeensis (Dugan et al., 2000). However, animals such as goats, dogs, and coyotes have also been identified as reservoirs which could play a limited role in the transmission of the pathogen to humans (Breitschwerdt et al., 1998; Dugan et al., 2000; Kocan et al., 2000). Unlike rickettsial species, ehrlichial species are not transmitted trans-ovarially (ie., larvae are uninfected) suggesting that the pathogen is maintained trans-stadially after the infection is acquired (Ismail et al., 2010). Although the reservoirs for E. chaffeensis in Cameroon have not yet been conclusively identified, preliminary studies detected antibodies reactive to E. chaffeensis in 56% of goats analysed suggesting a probable role of goats in maintaining the pathogen in nature. Moreover, E. chaffeensis DNA was detected in 17% of ticks collected from these animals (Ndip, unpubished data).
Fig. 2. Known distribution of ehrlichiae and rickettsiae in Cameroon
Clinical manifestations
The comprehensive data available in literature today on symptoms observed in HME infection is based on cases reported to the United States’ Centers for Disease Control and Prevention in addition to a series of patients studied since the disease was described. After exposure to an infecting tick, an incubation period of 1 to 2 weeks (median, 9 days) ensues after which patients develop a febrile illness (often >39°C) characterized by general malaise, low-back pain, or gastrointestinal symptoms (Paddock & Childs, 2003). These signs and symptoms most often resemble manifestations caused by other infectious and non-infectious causes. After 3 to 4 days, symptoms progress and patients may seek medical attention presenting with fever (>95%), headache (60 to 75%), myalgias (40 to 60%), nausea (40 to 50%), arthralgias (30 to 35%), and malaise (30 to 80%) (Fishbein et al., 1994). Some patients (10-40%) may present with cough, pharyngitis, diarrhea, or abdominal pain and may even progress to changes in mental status (Fishbein et al., 1994; Olano et al., 2003). Some populations especially HIV-infected patients (Paddock et al., 2001) and children (Jacobs & Schutze, 1997) may develop a rash on the extremities, trunk and face (Edwards, 1991). Hematological changes include leukopenia in approximately 60 to 70% of patients and thrombocytopenia (Fishbein et al., 1994; Olano & Walker, 2002). Liver enzymes (hepatic transaminases) may become slightly elevated (Nutt & Raufman, 1999). About 60 to 70% of patients require hospitalization and untreated cases last for 2-3 weeks or progress to a fatal outcome during the second week (Fishbein et al., 1994; Standaert et al., 2000). About 20% of patients develop neurologic signs, cough or other respiratory symptoms (Fishbein et al., 1994; Olano et al., 2003). Case-fatality ratio is approximately 3% (McQuiston et al., 2003) with risk factors for severe or fatal disease including older age (Paddock et al., 2001), underlying debilitating diseases such as HIV infection, immunosuppressive therapies (Olano & Walker, 2002) and sickle cell disease (Paddock & Childs, 2003).
These reported symptoms are quite similar to those manifested by Cameroonian HME patients. In one series of 206 acutely ill patients studied, 30 (14.6%) demonstrated anti- ehrlichial IgM antibodies, and these probable HME patients presented with headache (83%), fatigue (37%), abdominal pain (47%), joint pain (60%), anorexia (37%) and diarrhoea (13%) in addition to fever (>38oC). Their mean hematocrit, AST and ALT values were 48+21 %, 46±23% and 36+ 21%, respectively. Five (17%) of the patients were anaemic while 10 (33%) and 5 (17%) had abnormal AST and ALT values, respectively (Ndip, unpublished data). In another series of 118 acutely ill febrile patients studied with HME diagnosed by detection of E. chaffeensis DNA in patient’s blood (n=12), these patients presented with fever (100%), headache (seen in 72% of the patients), arthralgia (58%), myalgia (42%), cough (17%) and a diffuse maculopapular rash (17%). The rash was present on the trunk of one patient and the arms of another. One patient of the 12 with detectable E. chaffeensis DNA required hospitalization (see Table 1) (Ndip et al., 2009).
Epidemiology
The epidemiology and ecology of HME worldwide is not well documented. Since its description in 1986 more than 1000 cases of HME from at least 30 U.S. states have been reported to the Centers for Disease Control and Prevention in Atlanta, Georgia with nearly all occurring in the southeastern and south-central United States where the vector, A. americanum is common (Paddock & Childs, 2003; Dumler et al., 2007). However, the evidence of the disease and/or the pathogen is increasingly being reported in other parts of the world. This includes Africa (Uhaa et al., 1992; Brouqui et al., 1994; Ndip et al., 2009; Ndip et al., 2010), Israel (Dawson et al., 1991; Keysary et al., 1999; Brouqui & Dumler, 2000), Latin America (Gongora-Biachi et al., 1999; Calic et al., 2004;) and Asia (Heppner et al., 1997; Cao et al., 2000; Heo et al., 2002; Kim et al., 2003; Park et al., 2003, Lee & Chae, 2010).
In Cameroon, HME has been identified in patients along the coast of Cameroon, in Buea (4o10’0”N9o14’0”E), Limbe (4o01′N 9o13’13”E), Muyuka (4o43’18”N 9o38’27”E), Tiko (4o4’0”N 9o22’60”E), and Kumba (4o38’38” N9o26’19”E) and the agent, E. chaffeensis, in ticks collected from Limbe (Figure 2). HME was observed in both males and females as well as in children and adults although results suggested that older age was a risk factor for the disease (Ndip et al., 2009). The majority of the patients were adults which suggests that exposure to infected ticks may have occurred during outdoor activities such as farming. Another risk factor is that of owning a companion or domestic animal since most Cameroonian HME patients indicated they had tick-infested pets and domestic animals.
Microbiological diagnosis
The diagnosis of HME requires specialized microscopy equipment and skills which are not readily available in many diagnostic laboratories. Several methods have been proposed for the diagnosis of HME (Paddock & Childs, 2003; Ismail et al., 2010), including serologic tests such as immunofluorescent assay (IFA), western immunoblot employing specific proteins or ehrlichial whole cell antigens or the recently developed Ehrlichia recombinant protein or peptide ELISA for detection of the antibody (Cardenas et a!., 2007; Luo et al., 2010; O’Connor et al., 2010). Though these tests can be used to confirm diagnosis retrospectively, some patients may not sero-convert during the early days of the disease and cannot be diagnosed with serologic tests. However, collecting paired sera (at acute and convalescent phases of illness) is confirmatory as a four-fold rise in titer indicates current infection. However, this always presents a problem because patients who recover may not return to the hospital for follow up. Moreover, another issue with the interpretation of serological tests such as IFA is cross-reactive antibodies against other organisms, including Anaplasma species. PCR has also been employed to identify ehrlichial DNA in acutely ill patients when antibodies have not reached detectable levels. Several genes have been proposed and used including the VLPT gene (TRP32), TRP36, 16S rRNA, the TRP120, the Dsb, 28-kDa outer membrane protein gene have been used as genus or species specific targets (Yu et al., 1999; Doyle et al., 2005). IF A, western blot and PCR have been used to study the prevalence of ehrlichiae in blood of acutely ill patients, reservoirs, or in suspected tick vectors and anti-ehrlichial antibody in sera (Ndip et al., 2005; Ndip et al., 2007; Ndip et al., 2009; Ndip et al., 2010). Figure 3 shows IFA photomicrographs of whole cell of E. chaffeensis reacting with antibodies in an HME patient serum. A rapid method to detect E. chaffeensis is the observation of morulae in smears of peripheral blood buffy coat using the Diff Quik or Giemsa stain. However, this technique is very insensitive, and morulae are detected in leukocytes in only 10% of HME patients.
|
Patients |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
Gender |
F |
F |
M |
F |
M |
M |
F |
M |
F |
F |
M |
M |
|
Age (yr) |
63 |
40 |
5 |
23 |
22 |
20 |
21 |
1 |
16 |
26 |
35 |
25 |
|
Location |
A |
B |
C |
D |
C |
D |
A |
C |
A |
A |
B |
A |
|
Clinical |
||||||||||||
|
Manifestations |
||||||||||||
|
Fever >38oC |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
*Day(s) |
6 |
8 |
4 |
7 |
4 |
3 |
1 |
1 |
2 |
7 |
2 |
2 |
|
Headache |
Yes |
Yes |
Yes |
No |
No |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
No |
|
Myalgia |
Yes |
Yes |
No |
No |
No |
Yes |
No |
No |
Yes |
No |
Yes |
No |
|
Arthralgia |
Yes |
Yes |
No |
No |
No |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
No |
|
Rash |
No |
No |
No |
Yes |
No |
No |
No |
Yes |
No |
No |
No |
No |
*Days after onset (i.e., before collection of sample). Locations: A – Buea , B – Limbe, C – Tiko, D – Muyuka
Table 1. Epidemiologic and clinical characteristics of twelve Cameroonian patients with HME